Abstract
Purpose
Phase separation of trehalose during freeze-drying could render it ineffective as a lyoprotectant. The bulking agent, mannitol, on the other hand, should crystallize readily upon freezing. It is therefore imperative to understand the mutual interaction of these sugars during freezing to ensure preservation of the API during freeze-drying.
Methods
We investigated the effect of mannitol to trehalose ratio (R) on the crystallization behavior of both solutes using Differential Scanning Calorimetry, X-Ray Crystallography and FTIR Spectrosopy during controlled freezing and annealing.
Results
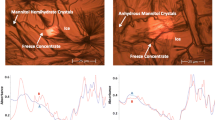
When R = 1, crystallization of both mannitol (as hemihydrate) and trehalose (as α-anhydrate) were observed. When R ≥ 1, extent of mannitol crystallization was directly proportional to the value of R. When R < 1, trehalose completely suppressed mannitol crystallization. At R > 1, the freeze concentrate was heterogeneous and characterized by two glass transitions – the lower temperature transition (Tg”) attributed to systems containing “extra” unfrozen water. When heated above Tg”, crystallization of mannitol and the associated unfrozen water resulted in Tg’ (glass transition temperature of the freeze-concentrate).
Conclusions
R and not the total solute concentration, dictates the composition of the freeze concentrate as well as the physical stability of the excipients





Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- DSC:
-
Differential scanning calorimetry
- FCL:
-
Freeze-concentrated liquid
- FTIR:
-
Fourier transform infrared spectroscopy
- IR:
-
Infrared
- XRD:
-
Powder X-ray Diffractometry
References
Carpenter JF, Pikal MJ, Chang BS, Randolph TW. Rational design of stable lyophilized protein formulations: some practical advice. Pharm Res. 1997;14(8):969–75.
K-i I, Yoshioka S, Terao T. Decreased protein-stabilizing effects of cryoprotectants due to crystallization. Pharm Res. 1993;10(8):1232–7.
Ken-ichi I, Sumie Y, Yasushi T. The effects of additives on the stability of freeze-dried β-galactosidase stored at elevated temperature. Int J Pharm. 1991;71(1):137–46.
Chatterjee K, Shalaev EY, Suryanarayanan R. Partially crystalline systems in lyophilization: II. Withstanding collapse at high primary drying temperatures and impact on protein activity recovery. J Pharm Sci. 2005;94(4):809–20.
Chatterjee K, Shalaev EY, Suryanarayanan R. Partially crystalline systems in lyophilization: I. Use of ternary state diagrams to determine extent of crystallization of bulking agent. J Pharm Sci. 2005;94(4):798–808.
Johnson RE, Kirchhoff CF, Gaud HT. Mannitol–sucrose mixtures—versatile formulations for protein lyophilization. J Pharm Sci. 2002;91(4):914–22.
Tang XC, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharm Res. 2004;21(2):191–200.
Wang DQ, Hey JM, Nail SL. Effect of collapse on the stability of freeze‐dried recombinant factor VIII and α‐amylase. J Pharm Sci. 2004;93(5):1253–63.
Zhou R, Schlam RF, Yin S, Gandhi RB, Adams ML. Scale considerations for selection of saccharide excipients for liquid formulations. J Pharm Sci. 2011;100(4):1605–6.
Varshney DB, Kumar S, Shalaev EY, Sundaramurthi P, Kang S-W, Gatlin LA, et al. Glycine crystallization in frozen and freeze-dried systems: effect of pH and buffer concentration. Pharm Res. 2007;24(3):593–604.
Varshney DB, Sundaramurthi P, Kumar S, Shalaev EY, Kang S-W, Gatlin LA, et al. Phase transitions in frozen systems and during freeze–drying: quantification using synchrotron X-Ray diffractometry. Pharm Res. 2009;26(7):1596–606.
Sundaramurthi P, Shalaev E, Suryanarayanan R. “pH swing” in frozen solutions consequence of sequential crystallization of buffer components. J Phys Chem Lett. 2009;1(1):265–8.
Szkudlarek BA. Selective crystallization of phosphate buffer components and pH changes during freezing: implications to protein stability: university of Michigan. 1997.
Carpenter JF, Prestrelski SJ, Arakawa T. Separation of freezing-and drying-induced denaturation of lyophilized proteins using stress-specific stabilization: I. Enzyme activity and calorimetric studies. Arch Biochem Biophys. 1993;303(2):456–64.
Piedmonte DM, Summers C, McAuley A, Karamujic L, Ratnaswamy G. Sorbitol crystallization can lead to protein aggregation in frozen protein formulations. Pharm Res. 2007;24(1):136–46.
Sundaramurthi P, Patapoff TW, Suryanarayanan R. Crystallization of trehalose in frozen solutions and its phase behavior during drying. Pharm Res. 2010;27(11):2374–83.
Sundaramurthi P, Shalaev E, Suryanarayanan R. Calorimetric and diffractometric evidence for the sequential crystallization of buffer components and the consequential pH swing in frozen solutions. J Phys Chem B. 2010;114(14):4915–23.
Sundaramurthi P, Suryanarayanan R. Trehalose crystallization during freeze-drying: implications on lyoprotection. J Phys Chem Lett. 2009;1(2):510–4.
Sundaramurthi P, Suryanarayanan R. Influence of crystallizing and non-crystallizing cosolutes on trehalose crystallization during freeze-drying. Pharm Res. 2010;27(11):2384–93.
Lu X, Pikal MJ. Freeze‐Drying of Mannitol–Trehalose–Sodium Chloride‐Based Formulations: The Impact of Annealing on Dry Layer Resistance to Mass Transfer and Cake Structure. Pharm Dev Technol. 2004;9(1):85–95.
Crowe JH, Crowe LM, Carpenter JF, Wistrom CA. Stabilization of dry phospholipid bilayers and proteins by sugars. Biochem J. 1987;242(1):1.
Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol. 1995;61(10):3592–7.
Sun WQ, Leopold AC, Crowe LM, Crowe JH. Stability of dry liposomes in sugar glasses. Biophys J. 1996;70(4):1769.
Esteves MI, Quintilio W, Sato RA, Raw I, De Araujo PS, Da Costa MHB. Stabilisation of immunoconjugates by trehalose. Biotechnol Lett. 2000;22(5):417–20.
Singh SK, Kolhe P, Mehta AP, Chico SC, Lary AL, Huang M. Frozen state storage instability of a monoclonal antibody: aggregation as a consequence of trehalose crystallization and protein unfolding. Pharm Res. 2011;28(4):873–85.
Liao X, Krishnamurthy R, Suryanarayanan R. Influence of processing conditions on the physical state of mannitol—implications in freeze-drying. Pharm Res. 2007;24(2):370–6.
Archer D. New NIST-traceable standards for calibration and validation of DSC. J Therm Anal Calorim. 2006;85(1):131–4.
Cavatur RK, Vemuri NM, Pyne A, Chrzan Z, Toledo-Velasquez D, Suryanarayanan R. Crystallization behavior of mannitol in frozen aqueous solutions. Pharm Res. 2002;19(6):894–900.
Burger A, Henck JO, Hetz S, Rollinger JM, Weissnicht AA, Stoettner H. Energy/temperature diagram and compression behavior of the polymorphs of D‐mannitol. J Pharm Sci. 2000;89(4):457–68.
Rudnick J, Taylor P, Litt M, Hopfinger A. Theory of free volume in polymers. J Polym Sci Polym Phys Ed. 1979;17(2):311–20.
Her L-M, Nail SL. Measurement of glass transition temperatures of freeze-concentrated solutes by differential scanning calorimetry. Pharm Res. 1994;11(1):54–9.
Pyne A, Surana R, Suryanarayanan R. Crystallization of mannitol below Tg′ during freeze-drying in binary and ternary aqueous systems. Pharm Res. 2002;19(6):901–8.
Kim AI, Akers MJ, Nail SL. The physical state of mannitol after freeze‐drying: Effects of mannitol concentration, freezing rate, and a noncrystallizing cosolute. J Pharm Sci. 1998;87(8):931–5.
Pyne A, Surana R, Suryanarayanan R. Enthalpic relaxation in frozen aqueous trehalose solutions. Thermochim Acta. 2003;405(2):225–34.
Powder Diffraction File. Hexagonal ice, card # 00-042-1142; D-trehalose dihydrate, card # 00-029-1955; trehalose anhydrate, card # 00-003-0312;β-D-mannitol, card # 00-022-1797; δ-D-mannitol, card # 00-022-1794. In.Powder Diffraction File. Newtown Square; 2004.
Toffel-Nadolny P. Infrared spectroscopic determinations of mannitol. Arch Kriminol. 1980;168(5–6):133–8.
Carpenter JF, Crowe JH. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry. 1989;28(9):3916–22.
Belton P, Gil A. IR and Raman spectroscopic studies of the interaction of trehalose with hen egg white lysozyme. Biopolymers. 1994;34(7):957–61.
Lin S-Y, Chien J-L. In vitro simulation of solid-solid dehydration, rehydration, and solidification of trehalose dihydrate using thermal and vibrational spectroscopic techniques. Pharm Res. 2003;20(12):1926–31.
Ragoonanan V, Aksan A. Heterogeneity in desiccated solutions: implications for biostabilization. Biophys J. 2008;94(6):2212–27.
Wolkers WF, Oliver AE, Tablin F, Crowe JH. A Fourier-transform infrared spectroscopy study of sugar glasses. Carbohydr Res. 2004;339(6):1077–85.
Nicolajsen H, Hvidt A. Phase behavior of the system trehalose-NaCl-water. Cryobiology. 1994;31(2):199–205.
Green JL, Angell CA. Phase relations and vitrification in saccharide-water solutions and the trehalose anomaly. J Phys Chem. 1989;93(8):2880–2.
Miller DP, de Pablo JJ, Corti H. Thermophysical properties of trehalose and its concentrated aqueous solutions. Pharm Res. 1997;14(5):578–90.
Mullin JW. Crystallization. Burlington: Elsevier Butterworth-Heinemann; 2001.
Luyet B, Rasmussen D. Study by differential thermal analysis of the temperatures of instability of rapidly cooled solutions of glycerol, ethylene glycol, sucrose and glucose. Biodynamica. 1967;10(210):167–91.
Liao X, Krishnamurthy R, Suryanarayanan R. Influence of the active pharmaceutical ingredient concentration on the physical state of mannitol—implications in freeze-drying. Pharm Res. 2005;22(11):1978–85.
Sussich F, Skopec C, Brady J, Cesàro A. Reversible dehydration of trehalose and anhydrobiosis: from solution state to an exotic crystal? Carbohydr Res. 2001;334(3):165–76.
Malsam J, Aksan A. Hydrogen bonding and kinetic/thermodynamic transitions of aqueous trehalose solutions at cryogenic temperatures. J Phys Chem B. 2009;113(19):6792–9.
Elias ME, Elias AM. Trehalose + water fragile system: properties and glass transition. J Mol Liq. 1999;83(1):303–10.
Kawai H, Sakurai M, Inoue Y, Chujo R, Kobayashi S. Hydration of oligosaccharides: anomalous hydration ability of trehalose. Cryobiology. 1992;29(5):599–606.
Magazu S, Migliardo P, Musolino AM, Sciortino MT. α, α-Trehalose-water solutions. 1. Hydration phenomena and anomalies in the acoustic properties. J Phys Chem B. 1997;101(13):2348–51.
Brown GM, Levy HA. α-D-glucose: precise determination of crystal and molecular structure by neutron-diffraction analysis. Science. 1965;147(3661):1038–9.
Brown GM, Rohrer DC, Berking B, Beevers CA, Gould RO, Simpson R. The crystal structure of α, α-trehalose dihydrate from three independent X-ray determinations. Acta Crystallogr Sect B: Struct Crystallogr Cryst Chem. 1972;28(11):3145–58.
Jeffrey GA. Intramolecular hydrogen-bonding in carbohydrate crystal-structures. Carbohydr Res. 1973;28(2):233–41.
Bennett AN, Bennett AR, Nees AR. Viscosity of beet house sirups. Ind Eng Chem. 1930;22(1):91–6.
Miller DP, de Pablo JJ. Calorimetric solution properties of simple saccharides and their significance for the stabilization of biological structure and function. J Phys Chem B. 2000;104(37):8876–83.
Km L, Labuza TP. Crystallization inhibition of an amorphous sucrose system using raffinose. J Zhejiang Univ (Sci). 2006;2:67–79.
Laos AK, Kirs BE, Kikkas CA, Paalme DT. Crystallization of the supersaturated sucrose solutions in the presence of fructose, glucose and corn syrup. In.; 2007. p. 16–20.
Leinen KM, Labuza TP. Crystallization inhibition of an amorphous sucrose system using raffinose. J Zhejiang Univ Sci B. 2006;7(2):85–9.
Smythe BM. Sucrose crystal growth. II. Rate of crystal growth in the presence of impurities. Aust J Chem. 1967;20(6):1097–114.
Laliberté M. Model for calculating the viscosity of aqueous solutions. J Chem Eng Data. 2007;52(2):321–35.
ACKNOWLEDGMENTS AND DISCLOSURES
This research was funded by an NSF grant (CBET-1335936) to A.A. The authors are grateful to Dr. Seema Thakral for guidance while performing the powder X-ray diffraction. Parts of this work were carried out in the Characterization Facility, University of Minnesota, which receives partial support from NSF through the MRSEC program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 313 kb)
Rights and permissions
About this article
Cite this article
Jena, S., Suryanarayanan, R. & Aksan, A. Mutual Influence of Mannitol and Trehalose on Crystallization Behavior in Frozen Solutions. Pharm Res 33, 1413–1425 (2016). https://doi.org/10.1007/s11095-016-1883-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1883-7