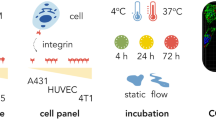
ABSTRACT
Purpose
Cell penetrating peptides (CPPs) can mediate effective delivery of their associated drugs and drug carriers intracellularly, however their lack of cell specificity remains a major obstacle for their clinical development. We aimed at improving the cell specificity and therapeutic efficacy of HPMA copolymer-octaarginine (R8) conjugate (P-R8) in cells at the tumor micro-environment.
Methods
To avoid premature cell-penetration, the positively charged R8 moieties were masked via electrostatic complexation with various polyanionic molecules (heparin sulfate, hyaluronic acid, fucoidan and poly-glutamic acid). We followed the kinetics of the FITC-labeled P-R8 penetration into endothelial and cancer cells over-time after its complexation in vitro and further tested whether the in situ addition of a stronger polycation can trigger the release of P-R8 from the complexes to resume cell penetration activity. A murine model of B16-F10 lung metastasis was then used as an in vivo model for assessing the therapeutic efficacy of the P-R8, loaded with doxorubicin (P-R8-DOX), after its complexation with PGA.
Results
The intracellular penetration of P-R8-FITC was reversibly inhibited by forming electrostatic interactions with counter polyanions, and can be restored either gradually over time by dissociation from the polyanions, or promptly following the addition of protamine sulfate. Mice injected with B16-F10 cells and treated with P-R8-DOX/PGA complexes, exhibited a significant prolonged survival times when compared with DOX-treated mice or relative to mice treated with either P-R8-DOX or P-DOX alone.
Conclusions
The gradual release of P-R8 from P-R8-DOX/PGA may improve the therapeutic efficacy of water-soluble based nanomedicines for the treatment of solid lung tumors.









Similar content being viewed by others
Abbreviations
- AIBN:
-
2,2’-azobis(isobutyronitrile)
- CPPs:
-
Cell-penetrating peptides
- DOX:
-
Doxorubicin
- EPR:
-
Enhanced permeability and retention
- Fi:
-
Fucoidan
- FITC:
-
Fluorescein-5-isothiocyanate
- HA:
-
Hyaluronic acid
- Hep:
-
Heparin
- HMW-Hep:
-
High molecular weight heparin
- HPMA:
-
N-(2-hydroxypropyl)methacrylamide
- LMW-Hep:
-
Low molecular weight heparin
- MTT:
-
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolinium bromide
- PFA:
-
Paraformaldehyde
- PGA:
-
Poly-(l)-glutamic acid
- PMA:
-
Poly(methacrylic acid)
- R8:
-
Octaarginine
- SEC:
-
Size-exclusion chromatography
REFERENCES
Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60.
Kopecek J, Kopeckova P. HPMA copolymers: origins, early developments, present, and future. Adv Drug Deliv Rev. 2010;62:122–49.
Egusquiaguirre SP, Igartua M, Hernandez RM, Pedraz JL. Nanoparticle delivery systems for cancer therapy: advances in clinical and preclinical research. Clin Transl Oncol. 2012;14:83–93.
Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–31.
Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Br J Cancer. 2008;99:392–7.
Khandare JJ, Minko T. Antibodies and peptides in cancer therapy. Crit Rev Ther Drug Carrier Syst. 2006;23:401–35.
Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–92.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84.
Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–503.
Brekken C, Bruland OS, de Lange Davies C. Interstitial fluid pressure in human osteosarcoma xenografts: significance of implantation site and the response to intratumoral injection of hyaluronidase. Anticancer Res. 2000;20:3503–12.
Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161:175–87.
Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–63.
David A. Carbohydrate-based biomedical copolymers for targeted delivery of anticancer drugs. Israel J Chem. 2010;50:204–19.
Karra N, Benita S. The ligand nanoparticle conjugation approach for targeted cancer therapy. Curr Drug Metab. 2012;13:22–41.
David A, Kopeckova P, Minko T, Rubinstein A, Kopecek J. Design of a multivalent galactoside ligand for selective targeting of HPMA copolymer-doxorubicin conjugates to human colon cancer cells. Eur J Cancer. 2004;40:148–57.
Shamay Y, Paulin D, Ashkenasy G, David A. E-selectin binding peptide-polymer-drug conjugates and their selective cytotoxicity against vascular endothelial cells. Biomaterials. 2009;30:6460–8.
Shamay Y, Paulin D, Ashkenasy G, David A. Multivalent display of quinic acid based ligands for targeting E-selectin expressing cells. J Med Chem. 2009;52:5906–15.
Adar L, Shamay Y, Journo G, David A. Pro-apoptotic peptide-polymer conjugates to induce mitochondrial-dependent cell death. Polym Adv Technol. 2011;22:199–208.
Kopansky E, Shamay Y, David A. Peptide-directed HPMA copolymer-doxorubicin conjugates as targeted therapeutics for colorectal cancer. J Drug Target. 2011;19:933–43.
Journo-Gershfeld G, Kapp D, Shamay S, Kopeček J, David A. Hyaluronan oligomers-HPMA copolymer conjugates for targeting paclitaxel to CD44-overexpressing ovarian Carcinoma. Pharm Res. 2012;29:1121–33.
Lindgren M, Hallbrink M, Prochiantz A, Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103.
Schwarze SR, Dowdy SF. In vivo protein transduction: intracellular delivery of biologically active proteins, compounds and DNA. Trends Pharmacol Sci. 2000;21:45–8.
Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J Control Release. 2007;118:216–24.
Kale AA, Torchilin VP. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: the effect of substitutes at the hydrazone linkage on the ph stability of PEG-PE conjugates. Bioconjug Chem. 2007;18:363–70.
Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci U S A. 2004;101:17867–72.
Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, et al. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol (Camb). 2009;1:382–93.
Shamay Y, Adar L, Ashkenasy G, David A. Light induced drug delivery into cancer cells. Biomaterials. 2011;32:1377–86.
Rihova B, Bilej M, Vetvicka V, Ulbrich K, Strohalm J, Kopecek J, et al. Biocompatibility of N-(2-hydroxypropyl) methacrylamide copolymers containing adriamycin. Immunogenicity, and effect on haematopoietic stem cells in bone marrow in vivo and mouse splenocytes and human peripheral blood lymphocytes in vitro. Biomaterials. 1989;10:335–42.
Kopecek J, Bazilova H. Poly[N-(2-hydroxypropyl)methacrylamide] I. Radical polymerization and copolymerization. Eur Polym J. 1973;9:7–14.
Drobník J, Kopeček J, Labsk J, Rejmanová P, Exner J, Saudek V. Enzymatic cleavage of side chains of synthetic water-soluble polymers. Makromol Chem. 1976;177:2833–48.
Omelyanenko V, Kopeckova P, Gentry C, Kopecek J. Targetable HPMA copolymer-adriamycin conjugates. Recognition, internalization, and subcellular fate. J Control Release. 1998;53:25–37.
Etrych T, Jelinkova M, Rihova B, Ulbrich K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: synthesis and preliminary in vitro and in vivo biological properties. J Control Release. 2001;73:89–102.
Seymour LW, Ulbrich K, Steyger PS, Brereton M, Subr V, Strohalm J, et al. Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma. Br J Cancer. 1994;70:636–41.
Kwon YM, Li Y, Naik S, Liang JF, Huang Y, Park YJ, et al. The ATTEMPTS delivery systems for macromolecular drugs. Expert Opin Drug Deliv. 2008;5:1255–66.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported in part by grants from the Israel Science Foundation (ISF) (Grant No. 418/10 to AD) and the Israeli National Nanotechnology Initiative for a Focal Technology Area (FTA) on Nanomedicines for Personalized Theranostics (to AD). GA acknowledges support from the E.J. Safra center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shamay, Y., Shpirt, L., Ashkenasy, G. et al. Complexation of Cell-Penetrating Peptide–Polymer Conjugates with Polyanions Controls Cells Uptake of HPMA Copolymers and Anti-tumor Activity. Pharm Res 31, 768–779 (2014). https://doi.org/10.1007/s11095-013-1198-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1198-x