Abstract
Purpose
The purpose of this study is to demonstrate that the erythroid precursor depletion in bone marrow induced by recombinant human erythropoietin (rHuEPO) treatment may be another contributing factor to erythropoietin hyporesponsiveness.
Methods
Healthy Wistar rats were given single dose (SD) or multiple doses (MD) of rHuEPO (100 IU/kg). In MD study, animals were challenged with thrice-weekly over two weeks. Blood, bone marrow and spleen (for SD only) were collected. The erythropoietic responses in bone marrow and spleen were quantified using a flow cytometric immunophenotyping technique. A mathematical approach involving measuring reticulocyte age distribution was developed to evaluate the reticulocyte loss due to neocytolysis.
Results
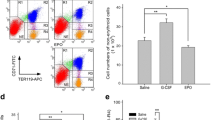
A reduced level of erythropoietic responses below the baseline was observed for both MD and SD studies. In SD study, the reticulocyte decreased below the baseline after day 6. A depletion of the bone marrow erythroid precursor cells was observed. However, neocytolysis of reticulocyte only occurs from day 3-5 after rHuEPO injection.
Conclusions
The findings demonstrate that EPO-induced erythroid precursor depletion in bone marrow is responsible for reduced reticulocyte response and may contribute to erythropoietin hyporesponsiveness. Therefore, this study provides further justification for reducing the doses of erythropoietin-stimulating agents in anemic patients demonstrating hyporesponsiveness.







Similar content being viewed by others
References
Koury MJ, Sawyer ST, Brandt SJ. New insights into erythropoiesis. Curr Opin Hematol. 2002;9(2):93–100.
Elliott S, Pham E, Macdougall IC. Erythropoietins: a common mechanism of action. Exp Hematol. 2008;36(12):1573–84.
Richmond TD, Chohan M, Barber DL. Turning cells red: signal transduction mediated by erythropoietin. Trends Cell Biol. 2005;15(3):146–55.
Koury MJ, Bondurant MC. Maintenance by erythropoietin of viability and maturation of murine erythroid precursor cells. J Cell Physiol. 1988;137(1):65–74.
Testa U. Apoptotic mechanisms in the control of erythropoiesis. Leukemia. 2004;18(7):1176–99.
Wintrobe MM, Greer JP. Wintrobe’s clinical hematology: Lippincott Williams & Wilkins; 2004.
Raff MC. Social controls on cell survival and cell death. Nature. 1992;356(6368):397–400.
Hillman RS. Characteristics of marrow production and reticulocyte maturation in normal man in response to anemia. J Clin Invest. 1969;48(3):443–53.
Major A, Bauer C, Breymann C, Huch A, Huch R. rh-erythropoietin stimulates immature reticulocyte release in man. Br J Haematol. 1994;87(3):605–8.
Rodak BF, Fritsma GA, Doig K. Hematology: clinical principles and applications. 3rd ed. St. Louis: Saunders Elsevier; 2007.
Chasis JA, Prenant M, Leung A, Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989;74(3):1112–20.
Waugh RE. Reticulocyte rigidity and passage through endothelial-like pores. Blood. 1991;78(11):3037–42.
Noble NA, Xu QP, Hoge LL. Reticulocytes II: reexamination of the in vivo survival of stress reticulocytes. Blood. 1990;75(9):1877–82.
Alfrey CP, Udden MM, Leach-Huntoon C, Driscoll T, Pickett MH. Control of red blood cell mass in spaceflight. J Appl Physiol. 1996;81(1):98–104.
Alfrey CP, Rice L, Udden MM, Driscoll TB. Neocytolysis: physiological down-regulator of red-cell mass. Lancet. 1997;349(9062):1389–90.
Trial J, Rice L, Alfrey CP. Erythropoietin withdrawal alters interactions between young red blood cells, splenic endothelial cells, and macrophages: an in vitro model of neocytolysis. J Investig Med. 2001;49(4):335–45.
Rice L, Alfrey CP. Modulation of red cell mass by neocytolysis in space and on Earth. Pflugers Arch. 2000;441(2–3 Suppl):R91–4.
Rice L, Ruiz W, Driscoll T, et al. Neocytolysis on descent from altitude: a newly recognized mechanism for the control of red cell mass. Ann Intern Med. 2001;134(8):652–6.
Risso A, Turello M, Biffoni F, Antonutto G. Red blood cell senescence and neocytolysis in humans after high altitude acclimatization. Blood Cells Mol Dis. 2007;38(2):83–92.
Trial J, Rice L. Erythropoietin withdrawal leads to the destruction of young red cells at the endothelial-macrophage interface. Curr Pharm Des. 2004;10(2):183–90.
Macdougall IC, Cooper AC. Erythropoietin resistance: the role of inflammation and pro-inflammatory cytokines. Nephrol Dial Transplant. 2002;17 Suppl 11:39–43.
Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339(9):584–90.
Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791–8.
Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363(12):1146–55.
Elliott J, Mishler D, Agarwal R. Hyporesponsiveness to erythropoietin: causes and management. Adv Chronic Kidney Dis. 2009;16(2):94–100.
Johnson DW, Pollock CA, Macdougall IC. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology (Carlton). 2007;12(4):321–30.
Kanbay M, Perazella MA, Kasapoglu B, Koroglu M, Covic A. Erythropoiesis stimulatory agent- resistant anemia in dialysis patients: review of causes and management. Blood Purif. 2010;29(1):1–12.
Kwack C, Balakrishnan VS. Managing erythropoietin hyporesponsiveness. Semin Dial. 2006;19(2):146–51.
Macdougall IC, Cooper AC. Hyporesponsiveness to erythropoietic therapy due to chronic inflammation. Eur J Clin Invest. 2005;35 Suppl 3:32–5.
van der Putten K, Braam B, Jie KE, Gaillard CA. Mechanisms of Disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Nephrol. 2008;4(1):47–57.
Parfrey PS. Erythropoietin-stimulating agents in chronic kidney disease: a response to hyporesponsiveness. Semin Dial. 2011;24(5):495–7.
Ait-Oudhia S, Scherrmann JM, Krzyzanski W. Time-dependent clearance and hematological pharmacodynamics upon erythropoietin multiple dosing in rats. Biopharm Drug Dispos. 2010;31(5–6):298–315.
Cheung WK, Goon BL, Guilfoyle MC, Wacholtz MC. Pharmacokinetics and pharmacodynamics of recombinant human erythropoietin after single and multiple subcutaneous doses to healthy subjects. Clin Pharmacol Ther. 1998;64(4):412–23.
Woo S, Krzyzanski W, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous administration in rats. J Pharmacol Exp Ther. 2006;319(3):1297–306.
Wiczling P, Ait-Oudhia S, Krzyzanski W. Flow cytometric analysis of reticulocyte maturation after erythropoietin administration in rats. Cytometry A. 2009;75(7):584–92.
Donohue DM, Reiff RH, Hanson ML, Betson Y, Finch CA. Quantitative measurement of the erythrocytic and granulocytic cells of the marrow and blood. J Clin Invest. 1958;37(11):1571–6.
Papayannopoulou T, Finch CA. On the in vivo action of erythropoietin: a quantitative analysis. J Clin Invest. 1972;51(5):1179–85.
Hermans MH, Opstelten D. In situ visualization of hemopoietic cell subsets and stromal elements in rat and mouse bone marrow by immunostaining of frozen sections. J Histochem Cytochem. 1991;39(12):1627–34.
Lok CN, Ponka P. Identification of an erythroid active element in the transferrin receptor gene. J Biol Chem. 2000;275(31):24185–90.
Liu Y, Pop R, Sadegh C, Brugnara C, Haase VH, Socolovsky M. Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood. 2006;108(1):123–33.
Wiczling P, Krzyzanski W. Method of determination of the reticulocyte age distribution from flow cytometry count by a structured-population model. Cytometry A. 2007;71(7):460–7.
Kato M, Kato Y, Sugiyama Y. Mechanism of the upregulation of erythropoietin-induced uptake clearance by the spleen. Am J Physiol. 1999;276(5 Pt 1):E887–95.
De Maria R, Testa U, Luchetti L, et al. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood. 1999;93(3):796–803.
Bugelski PJ, Nesspor T, Volk A, et al. Pharmacodynamics of recombinant human erythropoietin in murine bone marrow. Pharm Res. 2008;25(2):369–78.
Socolovsky M, Murrell M, Liu Y, Pop R, Porpiglia E, Levchenko A. Negative autoregulation by FAS mediates robust fetal erythropoiesis. PLoS Biol. 2007;5(10):e252.
Singbrant S, Russell MR, Jovic T, et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood. 2011;117(21):5631–42.
Piron M, Loo M, Gothot A, Tassin F, Fillet G, Beguin Y. Cessation of intensive treatment with recombinant human erythropoietin is followed by secondary anemia. Blood. 2001;97(2):442–8.
Locatelli F, Del Vecchio L. Erythropoiesis-stimulating agents in renal medicine. Oncologist. 2011;16 Suppl 3:19–24.
Acknowledgments and Disclosures
This work was supported by the National Institutes of Health Grant GM 57980
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, X., Ait-Oudhia, S. & Krzyzanski, W. Erythropoietin-Induced Erythroid Precursor Pool Depletion Causes Erythropoietin Hyporesponsiveness. Pharm Res 30, 1026–1036 (2013). https://doi.org/10.1007/s11095-012-0938-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0938-7