Abstract
Purpose
Originally approved for three times/week dosing, recombinant human erythropoietin (rhEPO) is now often used at weekly intervals. We have studied rhEPO in mice to better understand why the extended dosing interval retains efficacy.
Methods
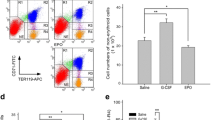
C57Bl/6 mice received a single sc. dose of rhEPO (3,000 IU/kg). Bone marrow and blood were collected at 8 h and 1, 2, 5 and 7 days. Staining for TER-119 and CD71, pulse labeling with bromodeoxyuridine, annexin-V binding and vital staining with 7-aminoactinomycin d were used cell cycle and apoptosis in erythroblasts by four color flow cytometry.
Results
A wave of proliferation and/or maturation progressed through all erythroblasts, resulting in the emigration of immature reticulocytes into the periphery. An increase in the fraction of erythroblasts in S and G2M was found, but suppression of apoptosis was not.
Conclusions
Most of the effects of rhEPO occurred 48 h after dosing, when the concentration of rhEPO was less than 1% of Cmax, suggesting that the processes set in motion by rhEPO can continue after rhEPO concentrations fall. Our observation of apoptosis in erythroblasts even when rhEPO concentrations were high suggests that regulatory mechanisms which down-regulate erythropoiesis are also engaged.








Similar content being viewed by others
References
M. J. Koury, S. T. Sawyer, and S. J. Brandt. New insights into erythropoiesis. Curr. Opinion. Hematol. 9:93–100 (2002).
D. Wen, J. P. Boissel, T. E. Tracy, et al. Erythropoietin structure–function relationships: high degree of sequence homology among mammals. Blood 82:1507–1516 (1993).
P. L. Pearson, T. P. Smith, T. S. Sonstegard, H. G. Klemcke, R. K. Christenson, and J. L. Vallet. Porcine erythropoietin receptor: molecular cloning and expression in embryonic and fetal liver. Domest. Anim. Endocrinol. 19:25–38 (2000).
J. L. Spivak. The mechanism of action of erythropoietin. Int. J. Cell Cloning. 4:139–166 (1986).
R. Maria De, U. Testa, and L. Luchetti. Apoptotic role of Fas/Fas ligand system in the regulation of erythropoiesis. Blood 93:796–803 (1999).
Y. Sadahira, and M. Mori. Role of macrophages in erythropoiesis. Pathol. Int. 49:841–848 (1999).
K.-H. Chang, M. Tam, and M. M. Stevenson. Inappropriately low reticulocytosis in severe malarial anemia correlates with suppression in the development of late erythroid precursors. Blood 103:3727–3735 (2004).
T. Kina, K. Ikuta, E. Takayama, K. Wada, A. S. Majumdar, I. L. Weissman, and Y. Katsura. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br. J. Haematol. 109:280–287 (2000).
M. Socolovsky, S. N. Constantinescu, S. Bergelson, A. Sirotkin, and H. F. Lodish. Cytokines in hematopoiesis: specificity and redundancy in receptor function. Adv. Protein Chem. 52:141–198 (1999).
W. Jelkmann. The enigma of the metabolic fate of circulating erythropoietin (Epo) in view of the pharmacokinetics of the recombinant drugs rhEpo and NESP. Eur. J. Haematol. 69:265–274 (2002).
B. Dalle, A. Henri, P. Rouyer-Fessard, et al. Dimeric erythropoietin fusion protein with enhanced erythropoietic activity in vitro and in vivo. Blood 97:3776–3782 (2001).
M. Kato, K. Miura, H. Kamiyama, et al. Pharmacokinetics of erythropoietin in genetically anemic mice. Drug Metab. Dispos. 26:126–131 (1998).
H. Bleuel, R. Hoffmann, B. Kaufmann, P. Neubert, P. P. Ochlich, and W. Schaumann. Kinetics of subcutaneous versus intravenous epoetin-beta in dogs, rats and mice. Pharmacology 52:329–338 (1996).
C. E. Lezon, M. P. Marinez, M. I. Conti, and C. E. Bozzini. Plasma disappearance of exogenous erythropoietin in mice under various experimental conditions. Endocrine 8:331–333 (1998).
O. Sowade, B. Sowade, K. Brilla, et al. Kinetics of reticulocyte maturity fractions and indices and iron status during therapy with epoetin beta (recombinant human erythropoietin) in cardiac surgery patients. Am. J. Hematol. 55:89–96 (1997).
D. Metcalf, and M. A. S Moore. Hematopoietic Cells. Elsevier, Amsterdam (1967).
J. Quinn, P. W. Fisher, R. J. Capocasale, et al. A statistical pattern recognition approach for determining cellular viability and lineage phenotype in cultured cells and murine bone marrow. Cytometry Part A (in press)
M. Holm, M. Thomsen, M. Hoyer, and P. Hokland. Optimization of a flow cytometric method for the simultaneous measurement of cell surface antigen, DNA content, and in vitro BrdUrd incorporation into normal and malignant hematopoietic cells. Cytometry 32:28–36 (1998).
S. H. Merchant, N. J. Gonchoroff, and R. E. Hutchison. Apoptotic index by annexin V flow cytometry: adjunct to morphologic and cytogenetic diagnosis of myelodysplastic syndromes. Cytometry 46:28–32 (2001).
V. Covelli, G. Briganti, and G. Silini. An analysis of bone marrow erythropoiesis in the mouse. Cell Tissue Kinet. 5:41–51 (1972).
B. I. Lord. Kinetics of the recognizable erythrocyte precursor cells. Clin. Hematol. 8:335–350 (1979).
Papayannopoulou T, Finch CA. On the in vivo action of erythropoietin: a quantitative analysis. J. Clin. Invest. 51:1179–1185 (1972).
J. C. Schooley. Responsiveness of hematopoietic tissue to erythropoietin in relation to the time of administration and duration of action of the hormone. Blood 25:795–808 (1965).
W. Nijhof, G. de Haan, J. Pietens, and B. Dontje. Mechanistic options of erythropoietin-stimulated erythropoiesis. Exp. Hematol. 23:369–375 (1995).
H. Borsook, J. B. Lingrel, J. L. Sears, and R. L. Millette. Synthesis of haemoglobin in relation to the maturation of erythroid cells. Nature 196:347–350 (1962).
G. J. Fruhman, and S. Fischer. The short-term effects of a single dose of erythropoietin upon reticulocytes in starved rats. Experimentia 18:462–464 (1962).
V. C. Broudy, N. Lin, M. Brice, B. Nakamoto, and T. Papayannopoulou. Erythropoietin receptor characteristics on primary human erythroid cells. Blood 77:2583–2590 (1991).
W. D. Lawrence, P. J. Davis, and S. D. Blas. Action of erythropoietin in vitro on rabbit reticulocyte membrane Ca2+-ATPase activity. J. Clin. Invest. 80:586–589 (1987).
S. M. Jacobs-Helber, and S. T. Sawyer. Jun N-terminal kinase promotes proliferation of immature erythroid cells and erythropoietin-dependent cell lines. Blood 104:696–703 (2004).
L. Glass, L. M. Lavidor, and S. H. Robinson. Use of cell separation and short-term culture techniques to study erythroid cell development. Blood 46:705–711 (1975).
G. D. Roodman, J. J. Hutton, and F. J. Bollum. DNA polymerase activities during erythropoiesis. Exp. Cell Res. 91:269–278 (1975).
E. Fibach, and E. A. Rachmilewitz. Stimulation of erythroid progenitors by high concentrations of erythropoietin results in normoblasts arrested in G2 phase of the cell cycle. Exp. Hematol. 21:184–188 (2003).
M. J. Koury, and M. C. Bondurant. Control of red cell production: roles of programmed cell death (apoptosis) and erythropoietin. Transfusion 8:673–674 (1990).
U. Testa. Apoptotic mechanisms in the control of erythropoiesis. Leukemia 18:1176–1199 (2004).
L. L. Kelley, M. J. Koury, M. C. Bondurant, S. T. Koury, S. T. Sawyer, and A. Wickrema. Survival or death of individual proerythroblasts results from differing erythropoietin sensitivities: a mechanism for controlled rates of erythrocyte production. Blood 82:2340–2352 (1993).
J. J. Brazil, and P. Gupta. Constitutive expression of the Fas receptor and its ligand in adult human bone marrow: a regulatory feedback loop for the homeostatic control of hematopoiesis. Blood Cells Mol. Diseases 29:94–103 (2002).
S. M. Jacobs-Helber, K.-H. Roh, D. Bailey, et al. Tumor necrosis-alpha expressed constitutively in erythroid cells or induced by erythropoietin has negative and stimulatory roles in normal erythropoiesis and erythroleukemia. Blood 101:524–531 (2003).
L. Zamai, S. Burattini, F. Luchetti, et al. In vitro apoptotic cell death during erythroid differentiation. Apoptosis 9:235–246 (2004).
C. H. Dai, J. O. Price, T. Brunner, and S. B. Krantz. Fas ligand is present on human erythroid colony-forming cells and interacts with Fas induced by interferon gamma to produce erythroid cell apoptosis. Blood 91:1235–1242 (1998).
M. Scharte, and M. P. Fink. Red blood cell physiology in critical illness. Crit. Care Med. 31(Suppl 12):S651–S657 (2003).
Perry C, and Soreq H. Transcriptional regulation of erythropoiesis. Fine tuning of combinatorial multi-domain elements. Eur. J. Biochem. 269:3607–3618 (2002).
L. Zamai, P. Secchiero, S. Pierpaoli, et al. TNF-related apoptosis-inducing ligand (TRAIL) as a negative regulator of normal human erythropoiesis. Blood 95:3716–3724 (2000).
M. Silva, C. Richard, A. Benito, C. Sanz, I. Olalla, J. L. Fernandez-Luna. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N. Eng. J. Med. 338:564–571 (1998).
K. Stahnke, S. Hecker, E. Kohne, K. M. Debatin. CD95 (APO-1/FAS)-mediated apoptosis in cytokine-activated hematopoietic cells. Exp. Hematol. 26:844–850 (1998).
Acknowledgements
This work was supported in its entirety by Centocor, a wholly owned subsidiary of Johnson & Johnson, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bugelski, P.J., Nesspor, T., Volk, A. et al. Pharmacodynamics of Recombinant Human Erythropoietin in Murine Bone Marrow. Pharm Res 25, 369–378 (2008). https://doi.org/10.1007/s11095-007-9372-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9372-7