ABSTRACT
Purpose
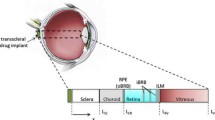
The objectives of this study were to determine the effects of permeant lipophilicity on permeant uptake into and transport across human sclera for transscleral delivery.
Methods
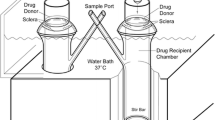
Model permeants with a wide range of lipophilicities were selected and studied with human sclera. Uptake experiments were carried out to measure permeant partitioning into the sclera. Transport experiments were performed in side-by-side diffusion cells, and the permeability coefficients and transport lag times of the permeants across the sclera were evaluated.
Results
Permeants with higher lipophilicity showed higher partition coefficients to human sclera, and the apparent transport lag time also increased significantly as the permeant lipophilicity increased. No correlation between the permeability coefficients and lipophilicity of the model permeants was observed in this study with human sclera. A hypothesis on the different findings between the present and previous studies was proposed.
Conclusions
Permeants with higher lipophilicity exhibited stronger binding to human sclera and would therefore lead to larger permeant partitioning to the sclera and longer transport lag time. The steady-state permeability coefficients of the permeants were not significantly affected by permeant lipophilicity.





Similar content being viewed by others
REFERENCES
Geroski DH, Edelhauser HF. Transscleral drug delivery for posterior segment disease. Adv Drug Deliv Rev. 2001;52:37–48.
Eljarrat-Binstock E, Pe’er J, Domb AJ. New techniques for drug delivery to the posterior eye segment. Pharm Res. 2010;27:530–43.
Pitkanen L, Ranta VP, Moilanen H, Urtti A. Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci. 2005;46:641–6.
Cheruvu NPS, Kompella UB. Bovine and porcine transscleral solute transport: influence of lipophilicity and the choroid-Bruch’s layer. Invest Ophthalmol Vis Sci. 2006;47:4513–22.
Myles ME, Neumann DM, Hill JM. Recent progress in ocular drug delivery for posterior segment disease: emphasis on transscleral iontophoresis. Adv Drug Deliv Rev. 2005;57:2063–79.
Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release. 2006;110:479–89.
Pontes de Carvalho RA, Krausse ML, Murphree AL, Schmitt EE, Campochiaro PA, Maumenee IH. Delivery from episcleral exoplants. Invest Ophthalmol Vis Sci. 2006;47:4532–9.
Cruysberg LP, Nuijts RM, Gilbert JA, Geroski DH, Hendrikse F, Edelhauser HF. In vitro sustained human transscleral drug delivery of fluorescein-labeled dexamethasone and methotrexate with fibrin sealant. Curr Eye Res. 2005;30:653–60.
Kadam RS, Kompella UB. Influence of lipophilicity on drug partitioning into sclera, choroid-retinal pigment epithelium, retina, trabecular meshwork, and optic nerve. J Pharmacol Exp Ther. 2010;332:1107–20.
Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci. 1998;87:1479–88.
Foster CS, de la Maza MS. Structural considerations of the sclera, in: The Sclera. New York: Springer-Verlag; 1994. p. 1–32.
Chopra P, Hao J, Li SK. Iontophoretic transport of charged macromolecules across human sclera. Int J Pharm. 2010;388:107–13.
Flynn GL, Yalkowsky SH, Roseman TJ. Mass transport phenomena and models: theoretical concepts. J Pharm Sci. 1974;63:479–510.
Chopra P, Hao J, Li SK. Effects of electroosmosis and electrophoresis in iontophoretic transport across human sclera, The 2008 AAPS Annual Meeting and Exposition, Atlanta, Georgia, 2008.
Li SK, Zhang Y, Zhu H, Higuchi WI, White HS. Influence of asymmetric donor-receiver ion concentration upon transscleral iontophoretic transport. J Pharm Sci. 2005;94:847–60.
Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems–recent advances. Prog Retin Eye Res. 1998;17:33–58.
Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007;14:507–15.
Cheong HI, Johnson J, Cormier M, Hosseini K. In vitro cytotoxicity of eight beta-blockers in human corneal epithelial and retinal pigment epithelial cell lines: comparison with epidermal keratinocytes and dermal fibroblasts. Toxicol In Vitro. 2008;22:1070–6.
Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128:289–96.
Shii D, Nakagawa S, Shinomiya K, Yoshimi M, Katsuta O, Oda T, et al. Cyclosporin A eye drops inhibit fibrosis and inflammatory cell infiltration in murine type I allergic conjunctivitis without affecting the early-phase reaction. Curr Eye Res. 2009;34:426–37.
Kacmaz RO, Kempen JH, Newcomb C, Daniel E, Gangaputra S, Nussenblatt RB, et al. Cyclosporine for ocular inflammatory diseases. Ophthalmology. 2010;117:576–84.
Thomas PA. Fungal infections of the cornea. Eye (London). 2003;17:852–62.
Habib FS, Fouad EA, Abdel-Rhaman MS, Fathalla D. Liposomes as an ocular delivery system of fluconazole: in-vitro studies. Acta Ophthalmol. in press.
Choritz L, Grub J, Wegner M, Pfeiffer N, Thieme H. Paclitaxel inhibits growth, migration and collagen production of human Tenon’s fibroblasts–potential use in drug-eluting glaucoma drainage devices. Graefes Arch Clin Exp Ophthalmol. 2010;248:197–206.
Wright SH, Wunz TM, Wunz TP. Structure and interaction of inhibitors with the TEA/H+ exchanger of rabbit renal brush border membranes. Pflugers Arch. 1995;429:313–24.
Lombardo F, Shalaeva MY, Tupper KA, Gao F. ElogD(oct): a tool for lipophilicity determination in drug discovery. 2. Basic and neutral compounds. J Med Chem. 2001;44:2490–7.
Frum Y, Bonner MC, Eccleston GM, Meidan VM. The influence of drug partition coefficient on follicular penetration: in vitro human skin studies. Eur J Pharm Sci. 2007;30:280–7.
Lucangioli SE, Kenndler E, Carlucci A, Tripodi VP, Scioscia SL, Carducci CN. Relation between retention factors of immunosuppressive drugs in microemulsion electrokinetic chromatography with biosurfactants and octanol-water partition coefficients. J Pharm Biomed Anal. 2003;33:871–8.
Moosavi-Movahedi AA, Hakimelahi S, Chamani J, Khodarahmi GA, Hassanzadeh F, Luo FT, et al. Design, synthesis, and anticancer activity of phosphonic acid diphosphate derivative of adenine-containing butenolide and its water-soluble derivatives of paclitaxel with high antitumor activity. Bioorg Med Chem. 2003;11:4303–13.
Geiser L, Henchoz Y, Galland A, Carrupt PA, Veuthey JL. Determination of pKa values by capillary zone electrophoresis with a dynamic coating procedure. J Sep Sci. 2005;28:2374–80.
Vincent-Ballereau FN, Patey ON, Lafaix C. Fluconazole. Review and situation among antifungal drugs in the treatment of opportunistic mycoses of human immuno-deficiency virus infections. Pharm Weekbl Sci. 1991;13:45–57.
ACKNOWLEDGEMENTS
This research was supported by NIH grant EY 015181. The authors acknowledge the use of tissues procured by the National Disease Research Interchange (NDRI) with support from NIH grant 5 U42 RR006042. The authors also thank Dr. Paul Bernstein and Moran Eye Center at the University of Utah for generously supplying us with some of the sclera tissues used in this study and Poonam Chopra for providing the transscleral transport data of tetraethylammonium, salicylate, and mannitol.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wen, H., Hao, J. & Li, S.K. Influence of Permeant Lipophilicity on Permeation Across Human Sclera. Pharm Res 27, 2446–2456 (2010). https://doi.org/10.1007/s11095-010-0237-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0237-0