Abstract
Purpose
To develop a pH-sensitive dexamethasone (Dex)-containing N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer conjugate with well-defined structure for the improved treatment of rheumatoid arthritis (RA).
Methods
A new pH-sensitive Dex-containing monomer (MA–Gly–Gly–NHN=Dex) was synthesized and copolymerized with HPMA using reversible addition–fragmentation transfer (RAFT) polymerization. The structure of the resulting HPMA copolymer–Dex conjugate (P-Dex) was analyzed and its therapeutic efficacy was evaluated on adjuvant-induced arthritis (AIA) rats.
Results
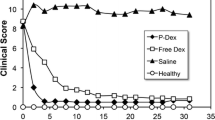
P-Dex was synthesized with controllable molecular weight and polydispersity index (PDI). The Dex content can be controlled by the feed-in ratio of MA–Gly–Gly–NHN=Dex. The P-Dex used for in vitro and in vivo evaluation has a average molecular weight (M w) of 34 kDa and a PDI of 1.34. The in vitro drug-release studies showed that the Dex release from the conjugate was triggered by low pH. Clinical measurements, endpoint bone mineral density (BMD) test and histology grading from the in vivo evaluation all suggest that newly synthesized P-Dex has strong and long-lasting anti-inflammatory and joint protection effects.
Conclusions
A HPMA copolymer–dexamethasone conjugate with a well-defined structure has been synthesized and proved to be an effective anti-arthritis therapy. It may have a unique clinical application in the treatment of rheumatoid arthritis.









Similar content being viewed by others
Abbreviations
- AI:
-
articular index
- AIA:
-
adjuvant-induced arthritis
- BMD:
-
bone mineral density
- DCC:
-
N,N′-dicyclohexylcarbodiimide
- DCU:
-
dicyclohexylurea
- Dex:
-
dexamethasone
- DMARDs:
-
disease-modifying anti-rheumatic drugs
- GCs:
-
glucocorticoids
- H & E:
-
hematoxylin and eosin
- HPMA:
-
N-(2-hydroxypropyl)methacrylamide
- LA:
-
N,N-dioctadecyl-N′,N′-bis(2-hydroxyethyl)propanediamine
- MA-FITC:
-
N-methacryloylaminopropyl fluorescein thiourea
- MA–Gly–Gly–OH:
-
N-methacryloylglycylglycine
- MA–Gly–Gly–NHN=Dex:
-
pH-sensitive Dex-containing monomer or N-(2-(2-(2-((8S,9R,10S,11S,13S,14S,16R,17R)-9-fluoro-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,11,12,13,15,16,17-octahydro-6H-cyclopenta[a]phenanthren-3(9H,10H,14H)-ylidene)hydrazinyl)-2-oxoethylamino)-2-oxoethyl)methacrylamide as generated by ChemDraw Ultra 9.0 (CambridgeSoft, Cambridge, MA, USA)
- M n :
-
number average molecular weight
- M w :
-
weight average molecular weight
- NSAIDs:
-
nonsteroidal anti-inflammatory drugs
- P-Dex:
-
copolymer of MA–Gly–Gly–NHN=Dex and HPMA
- pDEXA:
-
peripheral dual energy x-ray absorptiometry
- PDI:
-
polydispersity index
- SEC:
-
size exclusion chromatography
References
R. C. Lawrence, C. G. Helmick, F. C. Arnett, R. A. Deyo, D. T. Felson, E. H. Giannini, S. P. Heyes, K. Hirsh, M. C. Hochberg, G. G. Hunder, M. H. Liang, S. R. Pillemer, V. V. Steen, and F. Wolfe. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 41:778–799 (1998). doi:10.1002/1529-0131.
G. S. Firestein. Etiology and pathogenesis of rheumatoid arthritis. In Kelley’s Textbook of Rheumatology 7th edition. Edited by: E. D. Jr. Harris, R. C. Budd, M. C. Genovese, G. S. Firestein, J. S. Sargent, and C. B. Sledge. Philadelphia: Elsevier Saunders. 996–1042 (2005).
J. S. Smolen, and G. Steiner. Therapeutic strategies for rheumatoid arthritis. Nat. Rev. Drug Discov. 2:473–488 (2003). doi:10.1038/nrd1109.
J. R. O’Dell. Therapeutic strategies for rheumatoid arthritis. N. Engl. J. Med. 350:2591–2602 (2004). doi:10.1056/NEJMra040226.
G. A. FitzGerald. COX-2 and beyond: Approaches to prostaglandin inhibition in human disease. Nat. Rev. Drug Discov. 2:879–890 (2003). doi:10.1038/nrd1225.
D. J. Baylink. Glucocorticoid-induced osteoporosis. N. Engl. J. Med. 309:306–308 (1983).
A. T. Borchers, C. L. Keen, G. S. Cheema, and M. E. Gershwin. The use of methotrexate in rheumatoid arthritis. Semin. Arthritis Rheum. 34:465–483 (2004). doi:10.1016/j.semarthrit.2003.12.003.
N. J. Olsen, and C. M. Stein. New drugs for rheumatoid arthritis. N. Engl. J. Med. 350:2167–2179 (2004). doi:10.1056/NEJMra032906.
R. S. Traister, and R. Hirsch. Gene therapy for arthritis. Mod. Rheumatol. 18:2–14 (2008). doi:10.1007/s10165-007-0017-9.
H. Capell. Longterm maintenance therapy with disease modifying antirheumatic drugs. J. Rheumatol. Suppl. 66:38–43 (2002).
T. Garrood, and C. Pitzalis. Targeting the inflamed synovium: the quest for specificity. Arthritis Rheum. 54:1198–1208 (2006). doi:10.1002/art.21720.
O. C. Boerman, W. J. Oyen, G. Storm, M. L. Corvo, L. van Bloois, J. W. van der Meer, and F. H. Corstens. Technetium-99m labelled liposomes to image experimental arthritis. Ann. Rheum. Dis. 56:369–373 (1997).
D. Wang, S. C. Miller, M. Sima, D. Parker, H. Buswell, K. C. Goodrich, P. Kopečková, and J. Kopeček. The arthrotropism of macromolecules in adjuvant-induced arthritis rat model: a preliminary study. Pharm. Res. 21:1741–1749 (2004). doi:10.1023/B:PHAM.0000045232.18134.e9.
A. E. Koch. Angiogenesis as a target in rheumatoid arthritis. Ann. Rheum. Dis. 62(Suppl 2):ii60–67 (2003). doi:10.1136/ard.62.suppl_2.ii60.
Y. Matsumura, and H. Maeda. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46:6387–6392 (1986).
J. R. Levick. Permeability of rheumatoid and normal human synovium to specific plasma proteins. Arthritis Rheum. 24:1550–60 (1981). doi:10.1002/art.1780241215.
J. R. Levick. Hypoxia and acidosis in chronic inflammatory arthritis; relation to vascular supply and dynamic effusion pressure. J. Rheumatol. 17:579–582 (1990).
K. H. Falchuk, E. J. Goetzl, and J. P. Kulka. Respiratory gases of synovial fluids. An approach to synovial tissue circulatory–metabolic imbalance in rheumatoid arthritis. Am. J. Med. 49:223–231 (1970). doi:10.1016/S0002-9343(70)80078-X.
I. Goldie, and A. Nachemson. Synovial pH in rheumatoid knee-joints. I. The effect of synovectomy. Acta Orthop. Scand. 40:634–641 (1969).
S. E. Andersson, K. Lexmuller, A. Johansson, and G. M. Ekstrom. Tissue and intracellular pH in normal periarticular soft tissue and during different phases of antigen induced arthritis in the rat. J. Rheumatol. 26:2018–2024 (1999).
Y. T. Konttinen, J. Mandelin, T. F. Li, J. Salo, J. Lassus, M. Liljeström, M. Hukkanen, M. Takagi, I. Virtanen, and S. Santavirta. Acidic cysteine endoproteinase cathepsin K in the degeneration of the superficial articular hyaline cartilage in osteoarthritis. Arthritis Rheum. 46:953–960 (2002). doi:10.1002/art.10185.
Y. T. Konttinen, M. Takagi, J. Mandelin, J. Lassus, J. Salo, M. Ainola, T. F. Li, I. Virtanen, M. Liljeström, H. Sakai, Y. Kobayashi, T. Sorsa, R. Lappalainen, A. Demulder, and S. Santavirta. Acid attack and cathepsin K in bone resorption around total hip replacement prosthesis. J. Bone Miner. Res. 16:1780–1786 (2001). doi:10.1359/jbmr.2001.16.10.1780.
J. Schmidt, J. M. Metselaar, M. H. Wauben, K. V. Toyka, G. Storm, and R. Gold. Drug targeting by long-circulating liposomal glucocorticosteroids increases therapeutic efficacy in a model of multiple sclerosis. Brain. 126:1895–1904 (2003). doi:10.1093/brain/awg176.
J. M. Metselaar, M. H. Wauben, J. P. Wagenaar-Hilbers, O. C. Boerman, and G. Storm. Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum. 48:2059–66 (2003). doi:10.1002/art.11140.
J. M. Metselaar, W. B. Van den Berg, A. E. Holthuysen, M. H. Wauben, G. Storm, and P. L. van Lent. Liposomal targeting of glucocorticoids to synovial lining cells strongly increases therapeutic benefit in collagen type II arthritis. Ann. Rheum. Dis. 63:348–353 (2004). doi:10.1136/ard.2003.009944.
Y. Avnir, R. Ulmansky, V. Wasserman, S. Even-Chen, M. Broyer, Y. Barenholz, and Y. Naparstek. Amphipathic weak acid glucocorticoid prodrugs remote-loaded into sterically stabilized nanoliposomes evaluated in arthritic rats and in a Beagle dog: a novel approach to treating autoimmune arthritis. Arthritis Rheum. 58:119–129 (2008). doi:10.1002/art.23230.
D. Wang, S. C. Miller, X. M. Liu, B. Anderson, X. S. Wang, and S. R. Goldring. Novel dexamethasone–HPMA copolymer conjugate and its potential application in treatment of rheumatoid arthritis. Arthritis Res. Ther. 9:R2 (2007) doi:10.1186/ar2106".
J. T. Lai, D. Filla, and R. Shea. Functional polymers from novel carboxyl-terminated trithiocarbonates as highly efficient raft agents. Macromolecules. 35:6754–6756 (2002) doi:10.1021/ma020362m.
T. H. Cronin, H. Faubl, W. W. Hoffman, and J. J. Korst. Xylenediamines as antiviral agents. US patent 4,034,040, 1977.
J. Kopeček, and H. Bažilová. Poly[N-(2-hydroxypropyl)methacrylamide]. 1. Radical polymerization and copolymerization. Eur. Polym. J. 9:7–14 (1973) doi:10.1016/0014-3057(73)90063-3.
V. Omelyanenko, P. Kopečková, C. Gentry, and J. Kopeček. Targetable HPMA copolymer–adriamycin conjugates. Recognition, internalization, and subcellular fate. J. Control. Release. 53:25–37 (1998). doi:10.1016/S0168-3659(97)00235-6.
P. Rejmanová, J. Labský, and J. Kopeček. Aminolyses of monomeric and polymeric p-nitrophenyl esters of methacryloylated amino acids. Makromol. Chem. 178:2159–2168 (1977) doi:10.1002/macp.1977.021780803.
Y. H. Chang, C. M. Pearson, and C. Abe. Adjuvant polyarthritis. IV. Induction by a synthetic adjuvant: immunologic, histopathologic, and other studies. Arthritis Rheum. 23:62–71 (1980). doi:10.1002/art.1780230111.
C. F. van Dijke, C. G. Peterfy, R. C. Brasch, P. Lang, T. P. Roberts, D. Shames, J. B. Kneeland, Y. Lu, J. S. Mann, S. D. Kapila, and H. K. Genant. MR imaging of the arthritic rabbit knee joint using albumin-(Gd-DTPA)30 with correlation to histopathology. Magn. Reson. Imaging. 17:237–245 (1999). doi:10.1016/S0730-725X(98)00167-2.
M. S. Gordon, S. A. Sojka, and J. G. Krause. Carbon-13 NMR of para-substituted hydrazones, phenylhydrazones, oximes, and oxime methyl ethers: substituent effects on the iminyl carbon. J. Org. Chem. 49:97–100 (1984) doi:10.1021/jo00175a019.
M. V. Mirífico, J. A. Caram, and E. J. Vasini. The true configuration of the benzilosazone isomers. Tetrahedron Lett. 47:6919–6922 (2006) doi:10.1016/j.tetlet.2006.06.109.
C. Dugave, and L. Demange. Cis-trans isomerization of organic molecules and biomolecules: implications and applications. Chem. Rev. 103:2475–2532 (2003). doi:10.1021/cr0104375.
R. Duncan. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2:347–360 (2003). doi:10.1038/nrd1088.
F. Kratz, K. Abu Ajaj, and A. Warnecke. Anticancer carrier-linked prodrugs in clinical trials. Expert Opin. Investig. Drugs. 16:1037–58 (2007). doi:10.1517/13543784.16.7.1037.
R. Duncan. Designing polymer conjugates as lysosomotropic nanomedicines. Biochem. Soc. Trans. 35:56–60 (2007). doi:10.1042/BST0350056.
J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo, and S. H. Thang. Living free-radical polymerization by reversible addition–fragmentation chain transfer: the raft process. Macromolecules. 31:5559–5562 (1998) doi:10.1021/ma9804951.
M. J. Yanjarappa, K. V. Gujraty, A. Joshi, A. Saraph, and R. S. Kane. Synthesis of copolymers containing an active ester of methacrylic acid by RAFT: controlled molecular weight scaffolds for biofunctionalization. Biomacromolecules. 7:1665–1670 (2006). doi:10.1021/bm060098v.
H. Z. Pan, M. Sima, P. Kopečková, K. S. Wu, S. Q. Gao, J. H. Liu, D. Wang, S. C. Miller, and J. Kopeček. Biodistribution and pharmacokinetic studies of bone-targeting N-(2-Hydroxypropyl)methacrylamide copolymer–alendronate conjugates. Molecular Pharm. In press (2008).
J. R. Kirwan. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med. 333:142–146 (1995). doi:10.1056/NEJM199507203330302.
Acknowledgment
This work was supported in part by NIH grants AR053325 (DW) and AA10435 (GMT), the College of Pharmacy, University of Nebraska Medical Center and the UNeMed Corporation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xin-Ming Liu and Ling-Dong Quan have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, XM., Quan, LD., Tian, J. et al. Synthesis and Evaluation of a Well-defined HPMA Copolymer–Dexamethasone Conjugate for Effective Treatment of Rheumatoid Arthritis. Pharm Res 25, 2910–2919 (2008). https://doi.org/10.1007/s11095-008-9683-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9683-3