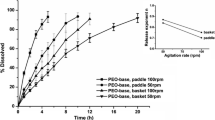
Abstract
Purpose
The objective was to investigate the feasibility of using a miniaturized disk intrinsic dissolution rate (IDR) apparatus to determine the Biopharmaceutics Classification System (BCS) solubility class, and to develop an approach where IDR measurements performed in media of different buffer capacity could be compared.
Methods
The disk IDR values of 14 model drugs were determined at 37°C in US Pharmacopeia buffers at pH 1.2, 4.5, and 6.8. As little as 5 mg of drug were compressed in a die, with surface area of 0.071 cm2, with the die assembly rotated at 100 rpm in 10 mL media. Drug concentration was measured by an in situ fiber optic ultraviolet method. The solubilities and pKas were determined, and used to simulate dissolution profiles with a convective-diffusion-with-chemical-reaction model.
Results
The disk IDR values spanned six orders of magnitude (0.00014 to 114 mg min−1 cm−2). The comparison of the miniaturized disk IDR values to published results using traditional dissolution bath apparatus indicated r 2 = 0.99.
Conclusions
The results demonstrate that using 100-fold less drug does not sacrifice the quality of the measurement, and lends support to an earlier study Yu et al. (Int. J. Pharm. 270:221–227, 2004) that the disk IDR measurement may possibly serve as a surrogate for the BCS solubility classification.








Similar content being viewed by others
References
Guidance for Industry. Waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms based on a Biopharmaceutics Classification System. FDA, Washington, D.C., 2000, August.
G. L. Amidon, H. Lennernäs, V. P. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420 (1995), doi:10.1023/A:1016212804288.
J. B. Dressman, R. R. Berardi, T. L. Dermentzoglou, S. P. Russell, J. L. Schmaltz, J. L. Barnett, and K. M. Jarvenpaa. Upper gastrointestinal (GI) pH in young healthy men and women. Pharm. Res. 7:756–761 (1990), doi:10.1023/A:1015827908309.
S. D. Mithani, V. Bakatselou, C. N. TenHoor, and J. B. Dressman. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm. Res. 13:163–167 (1996), doi:10.1023/A:1016062224568.
E. S. Kostewicz, M. Wunderlich, U. Brauns, R. Becker, T. Bock, and J. B. Dressman. Predicting the precipitation of poorly soluble weak bases upon entry in the small intestine. J. Pharm. Pharmacol. 56:43–51 (2004), doi:10.1211/0022357022511.
J. B. Dressman, G. L. Amidon, C. Reppas, and V. P. Shah. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm. Res. 15:11–22 (1998), doi:10.1023/A:1011984216775.
J. H. Wood, J. E. Syarto, and H. Letterman. Improved holder for disk intrinsic dissolution rate studies. J. Pharm. Sci. 54:1068 (1965), doi:10.1002/jps.2600540730.
A. S. Noyes, and W. R. Whitney. The rate of solution of solid substances in their own solutions. J. Amer. Chem. Soc. 19:930–934 (1897), doi:10.1021/ja02086a003.
K. G. Mooney, M. A. Mintun, K. J. Himmelstein, and V. J. Stella. Dissolution kinetics of carboxylic acids I: effect of pH under unbuffered conditions. J. Pharm. Sci. 70:13–22 (1981), doi:10.1002/jps.2600700103.
K. G. Mooney, M. A. Mintun, K. J. Himmelstein, and V. J. Stella. Dissolution kinetics of carboxylic acids II: effects of buffers. J. Pharm. Sci. 70:22–32 (1981), doi:10.1002/jps.2600700104.
A. T. M. Serajuddin, and C. I. Jarowski. pH-solubility profile of papaverine hydrochloride and its relationship to the dissolution rate of sustained-release pellets. J. Pharm. Sci. 73:1203–1208 (1984), doi:10.1002/jps.2600730905.
A. T. M. Serajuddin, and C. I. Jarowski. Effect of diffusion layer pH and solubility on the dissolution rate of pharmaceutical bases and their hydrochloride salts I: phenazopyridine. J. Pharm. Sci. 74:142–147 (1985), doi:10.1002/jps.2600740208.
D. P. McNamara, and G. L. Amidon. Dissolution of acidic and basic compounds from the rotating disk: influence of convective diffusion and reaction. J. Pharm. Sci. 75:858–868 (1986), doi:10.1002/jps.2600750907.
D. P. McNamara, and G. L. Amidon. Reaction plane approach for estimating the effects of buffers on the dissolution rate of acidic drugs. J. Pharm. Sci. 77:511–517 (1988), doi:10.1002/jps.2600770610.
M. Z. Southard, D. W. Green, V. J. Stella, and K. J. Himmelstein. Dissolution of ionizable drugs into unbuffered solution: a comprehensive model for mass transport and reaction in the rotating disk geometry. Pharm. Res. 9:58–69 (1992), doi:10.1023/A:1018979727118.
D. P. McNamara, M. L. Vieira, and J. R. Crison. Dissolution of pharmaceuticals in simple and complex systems. In G. L. Amidon, P. I. Lee, and E. M. Topp (eds.), Transport Processes in Pharmaceutical Systems, Marcel Dekker, New York, 2000, pp. 109–146.
J. Jinno, D. M. Oh, J. R. Crison, and G. L. Amidon. Dissolution of ionizable water-insoluble drugs: the combined effect of pH and surfactant. J. Pharm. Sci. 89:268–274 (2000), doi:10.1002/(SICI)1520-6017(200002)89:2<268::AID-JPS14>3.0.CO;2-F.
L. X. Yu, A. S. Carlin, G. L. Amidon, and A. S. Hussain. Feasibility studies of utilizing disk intrinsic dissolution rate to classify drugs. Int. J. Pharm. 270:221–227 (2004), doi:10.1016/j.ijpharm.2003.10.016.
S. Li, S. M. Wong, S. Sethia, H. Almoazen, Y. M. Joshi, and A. T. M. Serajuddin. Investigation of solubility of a free base and two different salt forms as a function of pH. Pharm. Res. 4:628–635 (2005), doi:10.1007/s11095-005-2504-z.
S. Li, P. Doyle, S. Metz, A. E. Royce, and A. T. M. Serajuddin. Effect of chloride ion on dissolution of different salt forms of haloperidol, a model basic drug. J. Pharm. Sci. 94:2224–2231 (2005), doi:10.1002/jps.20440.
J. J. Sheng, N. A. Kasim, R. Chandrasekharan, and G. L. Amidon. Solubilization and dissolution of insoluble weak acid, ketoprofen: effect of pH combined with surfactant. Eur. J. Pharm. Sci. 29:306–314 (2006), doi:10.1016/j.ejps.2006.06.006.
The United States Pharmacopeial Convention. United States Pharmacopeia (USP 23). The United States Pharmacopeial Convention, Rockville, 1995.
K. Bynum, K. Roinestad, A. Kassis, J. Pocreva, L. Gehriein, F. Cheng, and P. Palermo. Analytical performance of a fiber optic dissolution system. Dissol. Tech. 8:13–22 (2001).
V. A. Gray. Dissolution testing using fiber optics—a regulatory perspective. Amer. Pharm. Rev. 6:26–30 (2003).
C. J. Toher, P. E. Nielsen, A. S. Foreman, and A. Avdeef. In situ fiber optic dissolution monitoring of vitamin B12 solid dosage formulation. Dissolut. Tech. Nov:20–25 (2003).
A. Avdeef, D. Voloboy, and A. Foreman. Dissolution and solubility. In B. Testa, and H. van de Waterbeemd (eds.), Comprehensive Medicinal Chemistry II, Vol. 5 ADME-TOX Approaches, Elsevier, Oxford, 2006, pp. 399–423.
A. Avdeef. Solubility of sparingly-soluble drugs. In J. Dressman, and C. Reppas (Eds.) The Importance of Drug Solubility). Adv. Drug Deliv. Rev. 59:568–590 (2007), special issue, doi:10.1016/j.addr.2007.05.008.
C. M. Berger, O. Tsinman, D. Voloboy, D. Lipp, S. Stones, and A. Avdeef. Miniaturized intrinsic dissolution rate (Mini-IDR™) measurement of griseofulvin and carbamazepine. Dissolut. Tech. 14:39–41 (2007).
V. Bijlani, D. Yuonaye, S. Katpally, B. N. Chukwumezie, and M. C. Adeyeye. Monitoring ibuprofen release from multiparticulates: in situ fiber-optic technique versus the HPLC method. AAPS Pharm.Sci.Tech. 8, (2007) Article 52 (http://www.aapspharmscitech.org). doi:10.1208/pt0803052
A. M. Persson, K. Baumann, L. -O. Sundelöf, W. Lindberg, A. Sokolowski, and C. Pettersson. Design and characterization of a new miniaturized rotating disk equipment for in vitro dissolution rate studies. J. Pharm. Sci. 2008, in press. Published online Nov 2007.
D. D. Perrin, and B. Dempsey. Buffers for pH and Metal Ion Control. Chapman and Hall, London, 1974.
H. Wei, and R. Löbenberg. Biorelevant dissolution media as a predictive tool for glyburide a class II drug. Eur. J. Pharm. Sci. 26:45–52 (2006), doi:10.1016/j.ejps.2006.05.004.
S. Bendels, O. Tsinman, B. Wagner, D. Lipp, I. Parrilla, M. Kansy, and A. Avdeef. PAMPA-excipient classification gradient maps. Pharm. Res. 23:2525–2535 (2006), doi:10.1007/s11095-006-9137-8.
A. Avdeef. Absorption and Drug Development. Wiley, Hoboken, 2003.
A. Avdeef, and J. J. Bucher. Accurate measurements of the concentration of hydrogen ions with a glass electrode: calibrations using the Prideaux and other universal buffer solutions and a computer-controlled automatic titrator. Anal. Chem. 50:2137–2142 (1978), doi:10.1021/ac50036a045.
R. N. Jashnani, P. R. Byron, and R. N. Dalby. Validation of an improved Wood’s rotating disk dissolution apparatus. J. Pharm. Sci. 82:670–671 (1993), doi:10.1002/jps.2600820626.
H. Schlichting, and K. Gersten. Boundary Layer Theory, 8th ed. Springer, Berlin, 2000.
E. L. Cussler. Diffusion–Mass Transfer in Fluid Systems, 2nd ed. Cambridge Univ. Press, Cambridge, 1997, pp. 70–72.
V. G. Levich. Physiochemical Hydrodynamics. Prentice-Hall, Englewood Cliffs, 1962, pp. 39–72.
P. Bevington, and D. K. Robinson. Data Reduction and Error Analysis for the Physical Sciences, 3rd ed. McGraw-Hill, New York, 2002.
J. R. Vinograd, and J. W. McBain. Diffusion of electrolytes and of the ions in their mixture. J. Amer. Chem. Soc. 63:2008–2015 (1941), doi:10.1021/ja01852a063.
D. R. Olander. Simultaneous mass transfer and equilibrium chemical reaction. A.I.Ch.E. J. 6:233–239 (1960).
A. Fini, G. Fazio, and G. Feroci. Solubility and solubilization properties of non-steroidal antiinflammatory drugs. Int. J. Pharm. 126:95–102 (1995), doi:10.1016/0378-5173(95)04102-8.
A. Avdeef, S. Bendels, O. Tsinman, and M. Kansy. Solubility–excipient classification gradient maps. Pharm. Res. 24:530–545 (2007), doi:10.1007/s11095-006-9169-0.
A. Avdeef. Drug ionization and physicochemical profiling. In R. Mannhold (ed.), Drug Properties: Measurement and Computation, Wiley, Hoboken, 2007, pp. 55–83.
A. Avdeef, and J. Comer. A versatile potentiometric analyzer, part two: multiple known addition and Gran titration techniques. Amer. Lab. 19:116–124 (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Part 4 in the API-Sparing Dissolution Method series from pION. Berger et al. (28) is Part 3 in the series.
Rights and permissions
About this article
Cite this article
Avdeef, A., Tsinman, O. Miniaturized Rotating Disk Intrinsic Dissolution Rate Measurement: Effects of Buffer Capacity in Comparisons to Traditional Wood’s Apparatus. Pharm Res 25, 2613–2627 (2008). https://doi.org/10.1007/s11095-008-9679-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9679-z