Abstract
Purpose
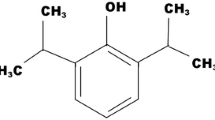
Propofol is a water-insoluble intravenous anesthetic agent that is actually formulated as a water-in-oil emulsion with known drawbacks such as pain on injection, microorganism growth support and stability. We report on the properties of formulations of propofol in poly (N-vinyl-2-pyrrolidone)-block-poly(d,l-lactide), PVP–PLA, polymeric micelles (Propofol-PM).
Methods
Microbial growth in these formulations was evaluated with Pseudomonas aeruginosa (ATCC 9027), Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 25922) and Candida albicans (ATCC 10231). Sleep-recovery studies in female Sprague–Dawley rats, at a dose of 10mg/kg were performed to compare pharmacodynamic profiles of the new Propofol-PM formulations with those of Diprivan®, a commercially available lipid based propofol formulation.
Results
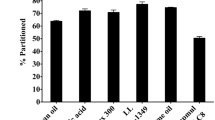
Growth of microorganisms was not supported in the Propofol-PM formulations tested. No significant differences in times to unconsciousness, awakening, recovery of righting reflex and full recovery were observed between Propofol-PM formulations and Diprivan®.
Conclusions
Propofol loaded in PVP–PLA micelles (Propofol-PM) is not significantly different in terms of pharmacodynamic but demonstrates no microorganism growth support and improved stability that opens up the door to pain on injection reduction strategy.





Similar content being viewed by others
References
J. B. Glen, and S. C. Hunter. Pharmacology of an emulsion formulation of ICI 35 868. Br. J. Anaesth. 56:617–626 (1984).
P. E. Marik. Propofol: therapeutic indications and side-effects. Curr. Pharm. Des. 10:3639–3649 (2004).
J. R. Sneyd. Recent advances in intravenous anaesthesia. Br. J. Anaesth. 93:725–736 (2004).
D. S. Ward, J. R. Norton, P. H. Guivarc’h, R. S. Litman, and P. L. Bailey. Pharmacodynamics and pharmacokinetics of propofol in a medium-chain triglyceride emulsion. Anesthesiology 97:1401–1408 (2002).
D. Song, M. A. Hamza, P. F. White, S. I. Byerly, S. B. Jones, and A. D. Macaluso. Comparison of a lower-lipid propofol emulsion with the standard emulsion for sedation during monitored anesthesia care. Anesthesiology 100:1072–1075 (2004).
D. Song, D. Hamza, P. F. White, K. Klein, A. Recart, and O. Khodaparast. The pharmacodynamic effects of a lower-lipid emulsion of propofol: a comparison with the standard propofol emulsion. Anesth. Analg. 98:687–691 (2004).
E. Kam, M. S. Abdul-Latif, and A. McCluskey. Comparison of Propofol-lipuro with propofol mixed with lidocaine 10mg on propofol injection pain. Anaesthesia. 59:1167–1169 (2004).
T. E. Morey, J. H. Modell, D. Shekhawat, T. Grand, D. O. Shah, N. Gravenstein, S. P. McGorray, and D. M. Dennis. Preparation and anesthetic properties of propofol microemulsions in rats. Anesthesiology 104:1184–1190 (2006).
H. Chen, Z. Zhang, O. Almarsson, J. F. Marier, D. Berkovitz, and C. R. Gardner. A novel lipid-free nanodispersion formulation of propofol and its characterization. Pharm. Res. 22:356–361 (2005).
T. D. Egan, S. E. Kern, K. B. Johnson, and N. L. Pace. The pharmacokinetics and pharmacodynamics of propofol in a modified cyclodextrin formulation (Captisol) versus propofol in a lipid formulation (Diprivan): an electroencephalographic and hemodynamic study in a porcine model. Anesth. Analg. 97:72–79 (2003).
A. Trapani, V. Laquintana, A. Lopedota, M. Franco, A. Latrofa, G. Talani, E. Sanna, G. Trapani, and G. Liso. Evaluation of new propofol aqueous solutions for intravenous anesthesia. Int. J. Pharm. 278:91–98 (2004).
P. K. Dubey, and A. Kumar. Pain on injection of lipid-free propofol and propofol emulsion containing medium-chain triglyceride: a comparative study. Anesth. Analg. 101:1060–1062 (2005).
M. T. Baker, and M. Naguib. Propofol: the challenges of formulation. Anesthesiology 103:860–876 (2005).
J. Fechner, H. Ihmsen, D. Hatterscheid, C. Jeleazcov, C. Schiessl, J. J. Vornov, H. Schwilden, and J. Schuttler. Comparative pharmacokinetics and pharmacodynamics of the new propofol prodrug GPI 15715 and propofol emulsion. Anesthesiology 101:626–639 (2004).
J. Fechner, H. Ihmsen, D. Hatterscheid, C. Schiessl, J. J. Vornov, E. Burak, H. Schwilden, and J. Schuttler. Pharmacokinetics and clinical pharmacodynamics of the new propofol prodrug GPI 15715 in volunteers. Anesthesiology 99:303–13 (2003).
M. G. Banaszczyk, A. T. Carlo, V. Millan, A. Lindsey, R. Moss, D. J. Carlo, and S. S. Hendler. Propofol phosphate, a water-soluble propofol prodrug: in vivo evaluation. Anesth. Analg. 95:1285–1292 (2002).
A. Benahmed, M. Ranger, and J. C. Leroux. Novel polymeric micelles based on the amphiphilic diblock copolymer poly(N-vinyl-2-pyrrolidone)-block-poly(d,l-lactide). Pharm. Res. 18:323–328 (2001).
D. Le Garrec, S. Gori, L. Luo, D. Lessard, D. C. Smith, M. A. Yessine, M. Ranger, and J. C. Leroux. Poly(N-vinylpyrrolidone)-block-poly(d,l-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. J. Control. Rel. 99:83–101 (2004).
L. Luo, M. Ranger, D. G. Lessard, D. Le Garrec, S. Gori, J. C. Leroux, S. Rimmer, and D. Smith. Novel amphiphilic diblock copolymer of low molecular weight poly(N-vinylpyrrolidone)-block-poly(d,l-lactide): synthesis, characterization, and micellization. Macromol. 37:4008–4013 (2004).
S. W. Provencher. A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 27:213–227 (1982).
J. W. Park, E. S. Park, S. C. Chi, H. Y. Kil, and K. H. Lee. The effect of lidocaine on the globule size distribution of propofol emulsions. Anesth. Analg. 97:769–771 (2003).
M. Yamakage, S. Iwasaki, J. I. Satoh, and A. Namiki. Changes in concentrations of free propofol by modification of the solution. Anesth. Analg. 101:385–388 (2005).
A. W. Doenicke, M. F. Roizen, J. Rau, W. Kellerman, and J. Balb. Reducing pain during propofol injection: the role of the solvent. Anesth. Analg. 82:472–474 (1996).
P. Picard, and M. R. Tramer. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth. Analg. 90:936–969 (2000).
C. B. Berry, T. Gillespie, J. Hood, and N. B. Scott. Growth of microorganisms in solutions of intravenous anaesthetic agents. Anaesthesia. 48:30–32 (1993).
S. N. Bennett, M. M. McNeil, L. A. Bland, and M. J. Arduino et al. Postoperative infections traced to contamination of an intravenous anesthetic propofol. N. Engl. J. Med. 333:147–154 (1995).
Information for Healthcare Professions Propofol (marketed as Diprivan and as generic products), United States Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) website: http://www.fda.gov/cder/drug/InfoSheets/HCP/propofolHCP.pdf
T. Fukada, M., and Ozaki. Microbial growth in propofol formulations with disodium edentate and the influence of venous access system dead space. Anesthesia 62:575–580 (2007).
Package insert. Diprivan®, 2006.
M. Tessler, A. Dascal, S. Gioseffini, M. Miller, and J. Mendelson. Growth curves of Staphylococcus aureus, Candida albicans, and Moraxella osloensis in propofol and other media. Can. J. Anaesth. 39:509–511 (1992).
Acknowledgements
The authors would like to thank Dr. Serge Messier for his expertise in reviewing and performing the microbial study protocol.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravenelle, F., Gori, S., Le Garrec, D. et al. Novel Lipid and Preservative-free Propofol Formulation: Properties and Pharmacodynamics. Pharm Res 25, 313–319 (2008). https://doi.org/10.1007/s11095-007-9471-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9471-5