No Heading
Purpose.
Propofol is a widely used anesthetic agent with highly desirable fast “on” and “off” effects. It is currently formulated as lipid emulsions, which are known to support microbial growth. In this study, a novel, lipid-free nanodispersion formulation of propofol was characterized.
Methods.
The formulation was evaluated for its physical and chemical stability, in vitro compatibility with red blood cells, and its antimicrobial effectiveness. In vivo pharmacokinetic and pharmacodynamic properties of the formulation were evaluated in rats.
Results.
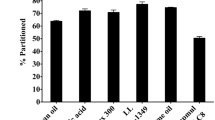
Our data suggest that this lipid-free formulation is physically and chemically stable. Compared to the commercial emulsion formulation Diprivan, it causes less hemolysis with red blood cells and has improved antimicrobial activity. In addition, the lipid-free formulation demonstrates similar pharmacological effects to Diprivan in rats.
Conclusions.
This novel, lipid-free formulation exhibits improved in vitro properties without compromising in vivo effects, therefore representing a promising new alternative for propofol.
Similar content being viewed by others
References
1. H. M. Bryson, B. R. Fulton, and D. Faulds. Propofol: an update on its use in anesthesia and conscious sedation. Drugs 50:513–559 (1995).
2. M. S. Langley and R. C. Heel. Propofol: a review of its pharmacodynamic and pharmacokinetic properties and use as an intravenous anesthetic. Drugs 33:334–372 (1988).
3. A. W. Doenicke, M. F. Roizen, J. Rau, M. O’Connor, J. Kugler, U. Klota, and J. Babl. Pharmacokinetics and pharmacodynamics of propofol in a new solvent. Anesth. Analg 85:1399–1403 (1997).
4. S. Dutta and W. F. Ebling. Emulsion formulation reduces propofol’s dose requirement and enhances safety. Anesthesiology 87:1394–1405 (1997).
5. K. McKeage and C. M. Perry. Propofol: a review of its use in intensive care sedation of adults. CNS Drugs 17:235–272 (2003).
6. W. Lindholm. Critically ill patients and fat emulsions. Minerva Anestesiol. 58:875–879 (1992).
7. S. Albrecht, H. Ihmsen, K. Suchodolski, C. Frenkel, and J. Achuttler. Analgo-sedation in intensive care: a quantitative, EEG-based trial with propofol 1% and 2%. Anaesthesist 48:794–801 (1999).
8. M. Schywalsky, H. Ihmsen, A. Tzabazis, J. Fechner, E. Burak, J. Vornov, and H. Schewilden. Pharmacokinetics and pharmacodynamics of the new propofol prodrug GPI 15715 in rats. Eur. J. Anaesthesiol. 20:182–190 (2003).
9. Diprivan® 1% injectable emulsion package insert. Available at http://www.astrazeneca-us.com/pi/202014Diprivan.pdf.
10. I. Wachowski, D. T. Jolly, J. Hrazdil, J. C. Galbraith, M. Greacen, and A. S. Clanachan. The growth of microorganisms in propofol and mixtures of propofol and lidocaine. Anesth. Analg. 88:209–212 (1999).
11. S. N. Bennett, M. M. McNeil, L. A. Bland, M. J. Arduino, M. E. Villarino, D. M. Perrotta, D. R. Burwen, S. F. Welbel, D. A. Pegues, and L. Stroud. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N. Engl. J. Med. 333:147–154 (1995).
12. J. Crowther, J. Hrazdil, D. T. Jolly, J. C. Galbraith, M. Greacen, and M. Grace. Growth of microorganisms in propofol, thiopental, and a 1:1 mixture of propofol and thiopental. Anesth. Analg. 82:475–478 (1996).
13. G. Trapani, A. Latrofa, M. Franco, A. Lopedota, E. Sanna, and E. Liso. Inclusion complexation of propofol with 2-hydroxypropyl-beta-cyclodextrin. Physicochemical, nuclear magnetic resonance spectroscopic studies, and anesthetic properties in rat. J. Pharm. Sci. 87:514 518–(1998).
14. J. Han, S. S. Davis, and C. Washington. Physical properties and stability of two emulsion formulations of propofol. Int. J. Pharm. 14:207–220 (2001).
15. H. Chen, Z. Zhang, C. McNulty, C. Olbert, H. J. Yoon, J. W. Lee, S. C. Kim, M. H. Seo, H. S. Oh, A. V. Lemmo, S. J. Ellis, and K. Heimlich. A high throughput combinatorial approach for the discovery of a Cremophor EL-free paclitaxel formulation. Pharm. Res. 20:1302–1308 (2003).
16. S. L. Morissette, Ö. Almarsson, M. L. Peterson, J. F. Remenar, M. J. Read, A. V. Lemmo, S. Ellis, M. J. Cima, and C. R. Gardner. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv. Drug Deliv. Rev. 56:275–300 (2004).
17. A. J. Bailer. Testing for the equality of area under the curves when using destructive measurement techniques. J. Pharmacokinet. Biopharm. 16:303–309 (1988).
18. M. T. Baker, M. S. Gregerson, S. M. Martin, and G. R. Buettner. Free radical and drug oxidation products in an intensive care unit sedative: propofol with sulfite. Crit. Care Med. 31:787–792 (2003).
19. J. J. Smith and B. E. Wayman. An evaluation of the antimicrobial effectiveness of citric acid as a root canal irrigant. J. Endod. 12:54–58 (1986).
20. M. Georgopoulou, E. Kontakiotis, and M. Nakou. Evaluation of the antimicrobial effectiveness of citric acid and sodium hypochlorite on the anaerobic flora of the infected root canal. Int. Endod. J. 27:139–143 (1994).
21. M. L. Veyries, F. Faurisson, M. L. Joly-Guillou, and B. Rouveix. Control of staphylococcal adhesion to polymethylmethacrylate and enhancement of susceptibility to antibiotics by poloxamer 407. Antimicrob. Agents Chemother. 44:1093–1096 (2000).
22. C. Jagannath, M. R. Emanuele, and R. L. Hunter. Activities of poloxamer CRL-1072 against Mycobacterium avium in macrophage culture and in mice. Antimicrob. Agents Chemother. 43:2898–2903 (1999).
23. M. Jumaa and B. W. Müller. In vitro investigation of the effect of various isotonic substances in parental emulsions on human erythrocytes. Eur. J. Pharm. Sci. 9:207 212 (1999).
24. I. D. Cockshott, E. J. Douglas, G. F. Plummer, and P. J. Simons. The pharmacokinetics of propofol in laboratory animals. Xenobiotica 22:369–375 (1992).
25. J. T. Chou and P. C. Jurs. Computation of partition coefficients from molecular structures by a fragment addition method. In S. H. Yalkowsky, A.A. Sinkula, and S.C. Valvani (eds.), Physical Chemical Properties of Drugs, Marcel Dekker, New York, 1980, pp. 163–199.
26. S. Dutta and W. F. Ebling. Formulation-dependent brain and lung distribution kinetics of propofol in rats. Anesthesiology 89:678–685 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Zhang, Z., Almarsson, Ö. et al. A Novel, Lipid-Free Nanodispersion Formulation of Propofol and Its Characterization. Pharm Res 22, 356–361 (2005). https://doi.org/10.1007/s11095-004-1872-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-004-1872-0