Abstract
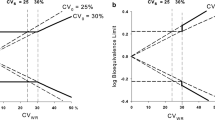
Over the past decade, concerns have been expressed increasingly regarding the difficulty for highly variable drugs and drug products (%CV greater than 30) to meet the standard bioequivalence (BE) criteria using a reasonable number of study subjects. The topic has been discussed on numerous occasions at national and international meetings. Despite the lack of a universally accepted solution for the issue, regulatory agencies generally agree that an adjustment of the traditional BE limits for these drugs or products may be warranted to alleviate the resource burden of studying relatively large numbers of subjects in bioequivalence trials. This report summarizes a careful examination of all the statistical methods available and extensive simulations for BE assessment of highly variable drugs/products. Herein, the authors present an approach of scaling an average BE criterion to the within-subject variability of the reference product in a crossover BE study, together with a point-estimate constraint imposed on the geometric mean ratio between the test and reference products. The use of a reference-scaling approach involves the determination of variability of the reference product, which requires replication of the reference treatment in each individual. A partial replicated-treatment design with this new data analysis methodology will thus provide a more efficient design for BE studies with highly variable drugs and drug products.
Similar content being viewed by others
References
US Code of Federal Regulations Title 21, Section 320.1(e). US Government Printing Office, Washington, DC, 2006.
US Food and Drug Administration. Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations, March 2003.
S. D. Patterson, N. M.-D. Zariffa, T. H. Montague, and K. Howland. Non-traditional study designs to demonstrate average bioequivalence for highly variable drug products. Eur. J. Pharm. Sci. 57:663–670 (2001).
L. Z. Benet. Therapeutic Considerations of Highly Variable Drugs. Meeting of FDA Committee for Pharmaceutical Science, October 6, 2006. http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4241s2_2_files/frame.htm (accessed 04/04/2007).
D. J. Schuirmann. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J. Pharmacokinet. Biopharm. 15:657–680 (1987).
U. S. Food and Drug Administration. Guidance for Industry: Statistical Approaches to Establishing Bioequivalence, January 2001.
FDA Orange Book, Preface p. viii. http://www.fda.gov/cder/ob/default.htm (Accessed 03/16/2007).
H. Blume and K. K. Midha. Report of Consensus Meeting: Bio-international ’92. Conference on Bioavailability, Bioequivalence and Pharmacokinetic Studies. Bad Homburg, Germany, 20–22 May, 1992. Eur. J. Pharm. Sci. 1:165–171 (1993).
Bio-international ’94, Munich Germany. 14–18 June, 1994.
B. M. Davit. Highly variable drugs—bioequivalence issues: FDA proposal under consideration. Meeting of FDA Committee for Pharmaceutical Science, October 6, 2006. http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4241s2_5.htm (accessed 3/12/2007).
Health Canada, Therapeutic Products Directorate (TPD). Discussion paper on ‘Bioequivalence requirements—highly variable drugs and highly variable drug products: issues and options’. Expert Advisory Committee on Bioavailability and Bioequivalence (EAC-BB) Meeting, June 26–27, 2003.
Health Canada, Ministry of Health. Guidance for Industry: Conduct and Analysis of Bioavailability and Bioequivalence Studies—Part A: Oral Dosage Formulations Used for Systemic Effects. 1992.
Committee for Proprietary Medicinal Products (CPMP), the European Agency for the Evaluation of Medicinal Products (EMEA). Note for Guidance on the Investigation of Bioavailability and Bioequivalence. 2001.
Japan National Institute of Health, Division of Drugs. Guideline for Bioequivalence Studies of Generic Drug Products. 1997.
FDA Advisory Committee on Pharmaceutical Science. April 13–14, 2004. http://www.fda.gov/ohrms/dockets/ac/04/minutes/4034M1.htm (accessed April 5, 2007).
A. W. Boddy, F. C. Snikeris, R. O. Kringle, G. C. G. Wei, J. A. Opperman, and K. K. Midha. An approach for widening the bioequivalence acceptance limits in the case of highly variable drugs. Pharm. Res. 12:1865–1868 (1995).
L. Tothfalusi and L. Endrenyi. Limits for the scaled average bioequivalence of highly variable drugs and drug products. Pharm. Res. 20:382–389 (2003).
L. Tothfalusi, L. Endrenyi, and K. K. Midha. Scaling or wider bioequivalence limits for highly variable drugs and for the special case of C max. Int. J. Clin. Pharmacol. Ther. 41:217–225 (2003).
L. Tothfalusi, L. Endrenyi, K. K. Midha, M. J. Rawson, and J. W. Hubbard. Evaluation of the bioequivalence of highly variable drugs and drug products. Pharm. Res. 18:728–733 (2001).
V. Karalis, M. Symillides, and P. Macheras. Novel scaled average bioequivalence limits based on GMR and variability considerations. Pharm. Res. 21:1933–1942 (2004).
S. H. Haidar. Evaluation of the scaling approach for highly variable drugs. Meeting of FDA Committee for Pharmaceutical Science, October 6, 2006. http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4241s2_4.htm (accessed 3/14/2007).
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions expressed in this report by the authors do not necessarily reflect the views or policies of the Food and Drug Administration (FDA).
Rights and permissions
About this article
Cite this article
Haidar, S.H., Davit, B., Chen, ML. et al. Bioequivalence Approaches for Highly Variable Drugs and Drug Products. Pharm Res 25, 237–241 (2008). https://doi.org/10.1007/s11095-007-9434-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9434-x