Abstract
Purpose
To elucidate and compare the activity and mechanism of albumin uptake in primary cultured alveolar type II and type I-like epithelial cells.
Materials and methods
Type II epithelial cells isolated from rat lungs were cultured for 2 days at 5 × 106 cells/35-mm dish or for 6 days at 2 × 106 cells/35-mm dish. The mRNA expression of marker genes and FITC-albumin uptake were examined.
Results
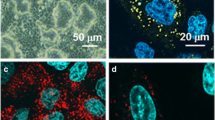
The cells cultured for 2 days exhibited cuboidal type II epithelial morphology with lamellar bodies inside the cells, while the cells cultured for 6 days exhibited squamous type I epithelial morphology. These morphological characteristics were consistent with the changes in mRNA expression pattern of marker genes. FITC-albumin uptake in both cells was temperature-dependent and was inhibited by metabolic inhibitors and bafilomycin A1. The rate of uptake was much higher in type II cells than type I-like cells. In both cells, FITC-albumin uptake was inhibited by clathrin mediated-endocytosis inhibitors, but not by caveolae mediated-endocytosis inhibitors.
Conclusions
These findings indicate that albumin in alveolar lining fluid is internalized into type II and type I epithelial cells via clathrin-mediated endocytosis, and the rate of albumin uptake is higher in type II cells than type I cells.







Similar content being viewed by others
Abbreviations
- ANOVA:
-
analysis of variance
- BAF:
-
bafilomycin A1
- CINC-1:
-
chemokine-induced neutrophilic chemoattractant-1
- CPZ:
-
chlorpromazine
- DMEM/F-12:
-
Dulbecco’s modified Eagle medium-nutrient mixture F-12 (1:1)
- DMSO:
-
dimethyl sulfoxide
- DNP:
-
2,4-dinitrophenol
- 2DOG:
-
2-deoxy-D-glucose
- EDTA:
-
ethylenediaminetetraacetic acid
- FBS:
-
fetal bovine serum
- FITC-albumin:
-
fluorescein isothiocyanate-labeled bovine serum albumin
- GAPDH:
-
glyceraldehyde-3-phosphate dehydrogenase
- IGFBP6:
-
insulin-like growth factor binding protein 6
- IND:
-
indomethacin
- MCD:
-
methyl-β-cyclodextrin
- mdr1a:
-
multidrug resistance protein 1a
- NaN3 :
-
sodium azide
- NYS:
-
nystatin
- PAO:
-
phenylarsine oxide
- PBS:
-
phosphate-buffered saline
- RTI40:
-
rat type I cell 40-kDa protein
- RT-PCR:
-
reverse transcription-polymerase chain reaction
- SDS:
-
sodium dodecyl sulfate
- SP-B:
-
surfactant protein B
References
J. S. Patton. Mechanisms of macromolecule absorption by the lungs. Adv. Drug Deliv. Rev. 19:3–36 (1996).
H. Fehrenbach. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir. Res. 2:33–46 (2001).
R. H. Hastings, H. G. Folkesson, and M. A. Matthay. Mechanisms of alveolar protein clearance in the intact lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L679–L689 (2004).
K. J. Kim and A. B. Malik. Protein transport across the lung epithelial barrier. Am. J. Physiol. Lung Cell. Mol. Physiol. 284:L247–L259 (2003).
T. A. John, S. M. Vogel, R. D. Minshall, K. Ridge, C. Tiruppathi, and A. B. Malik. Evidence for the role of alveolar epithelial gp60 in active transalveolar albumin transport in the rat lung. J. Physiol. 533:547–559 (2001).
R. Yumoto, H. Nishikawa, M. Okamoto, H. Katayama, J. Nagai, and M. Takano. Clathrin-mediated endocytosis of FITC-albumin in alveolar type II epithelial cell line RLE-6TN. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L946–L955 (2006).
M. Bur, H. Huwer, C. M. Lehr, N. Hagen, M. Guldbrandt, K. J. Kim, and C. Ehrhardt. Assessment of transport rates of proteins and peptides across primary human alveolar epithelial cell monolayers. Eur. J. Pharm. Sci. 28:196–203 (2006).
L. G. Dobbs. Isolation and culture of alveolar type II cells. Am. J. Physiol. 258:L134–L147 (1990).
J. M. Cheek, M. J. Evans, and E. D. Crandall. Type I cell-like morphology in tight alveolar epithelial monolayers. Exp. Cell Res. 184:375–387 (1989).
A. Steimer, E. Haltner, and C. M. Lehr. Cell culture models of the respiratory tract relevant to pulmonary drug delivery. J. Aerosol Med. 18:137–182 (2005).
R. J. Richards, N. Davies, J. Atkins, and V. I. C. Oreffo. Isolation, biochemical characterization, and culture of lung type II cells of the rat. Lung 165:143–158 (1987).
S. H. Hansen, K. Sandvig, and B. van Deurs. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121:61–72 (1993).
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275 (1951).
C. F. Cesarone, C. Bolognesi, and L. Santi. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal. Biochem. 100:188–197 (1979).
R. Gonzalez, Y. H. Yang, C. Griffin, L. Allen, Z. Tigue, and L. Dobbs. Freshly isolated rat alveolar type I cells, type II cells, and cultured type II cells have distinct molecular phenotypes. Am. J. Physiol. Lung Cell. Mol. Physiol. 288:L179–L189 (2005).
M. C. McElroy and M. Kasper. The use of alveolar epithelial type I cell-selective markers to investigate lung injury and repair. Eur. Respir. J. 24:664–673 (2004).
L. J. Reynolds, M. McElroy, and R. J. Richards. Density and substrata are important in lung type II cell transdifferentiation in vitro. Int. J. Biochem. Cell Biol. 31:951–960 (1999).
B. E. Goodman and E. D. Crandall. Dome formation in primary cultured monolayers of alveolar epithelial cells. Am. J. Physiol. 243:C96–C100 (1982).
T. R. Downs and W. W. Wilfinger. Fluorometric quantification of DNA in cells and tissue. Anal. Biochem. 131:538–547 (1983).
A. Chander, R. G. Johnson, J. Reicherter, and A. B. Fisher. Lung lamellar bodies maintain an acidic internal pH. J. Biol. Chem. 261:6126–6131 (1986).
N. Nelson and W. R. Harvey. Vacuolar and plasma membrane proton-adenosinetriphosphatases. Physiol. Rev. 79:361–385 (1999).
Y. Sasaki, J. Nagai, Y. Kitahara, N. Takai, T. Murakami, and M. Takano. Expression of chloride channel, ClC-5, and its role in receptor-mediated endocytosis of albumin in OK cells. Biochem. Biophys. Res. Commun. 282:212–218 (2001).
M. Takano, Y. Koyama, H. Nishikawa, T. Murakami, and R. Yumoto. Segment-selective absorption of lysozyme in the intestine. Eur. J. Pharmacol. 502:149–155 (2004).
Y. Matsukawa, H. Yamahara, F. Yamashita, V. H. Lee, E. D. Crandall, and K. J. Kim. Rates of protein transport across rat alveolar epithelial cell monolayers. J. Drug Target. 7:335–342 (2000).
K. J. Kim, Y. Matsukawa, H. Yamahara, V. K. Kalra, V. H. Lee, and E. D. Crandall. Absorption of intact albumin across rat alveolar epithelial cell monolayers. Am. J. Physiol. Lung Cell. Mol. Physiol. 284:L458–L465 (2003).
S. D. Conner and S. L. Schmid. Regulated portals of entry into the cell. Nature 422:37–44 (2003).
S. A. Mousavi, L. Malerod, T. Berg, and R. Kjeken. Clathrin-dependent endocytosis. Biochem. J. 377:1–16 (2004).
M. Kirkham and R. G. Parton. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim. Biophys. Acta. 1745:273–286 (2005).
L. H. Wang, K. G. Rothberg, and R. G. Anderson. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107–1117 (1993).
P. Ruckert, S. R. Bates, and A. B. Fisher. Role of clathrin- and actin-dependent endocytotic pathways in lung phospholipid uptake. Am. J. Physiol. Lung Cell. Mol. Physiol. 284:L981–L989 (2003).
C. C. Visser, S. Stevanovic, L. Heleen Voorwinden, P. J. Gaillard, D. J. Crommelin, M. Danhof, and A. G. De Boer. Validation of the transferrin receptor for drug targeting to brain capillary endothelial cells in vitro. J. Drug Target. 12:145–150 (2004).
S. B. Sieczkarski and G. R. Whittaker. Dissecting virus entry via endocytosis. J. Gen. Virol. 83:1535–1545 (2002).
H. R. Kim, S. Gil, K. Andrieux, V. Nicolas, M. Appel, H. Chacun, D. Desmaele, F. Taran, D. Georgin, and P. Couvreur. Low-density lipoprotein receptor-mediated endocytosis of PEGylated nanoparticles in rat brain endothelial cells. Cell. Mol. Life Sci. 64:356–364 (2007).
L. Campbell, A. J. Hollins, A. Al-Eid, G. R. Newman, C. von Ruhland, and M. Gumbleton. Caveolin-1 expression and caveolae biogenesis during cell transdifferentiation in lung alveolar epithelial primary cultures. Biochem. Biophys. Res. Commun. 262:744–751 (1999).
E. I. Christensen and H. Birn. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am. J. Physiol. Renal Physiol. 280:F562–F573 (2001).
E. I. Christensen and H. Birn. Megalin and cubilin: multifunctional endocytic receptors. Nat. Rev. Mol. Cell Biol. 3:258–268 (2002).
M. G. Farquhar, A. Saito, D. Kerjaschki, and R. A. Orlando. The Heymann nephritis antigenic complex: megalin (gp330) and RAP. J. Am. Soc. Nephrol. 6:35–47 (1995).
J. Nagai and M. Takano. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab. Pharmacokinet. 19:159–170 (2004).
J. Nagai, H. Tanaka, N. Nakanishi, T. Murakami, and M. Takano. Role of megalin in renal handling of aminoglycosides. Am. J. Physiol. Renal Physiol. 281:F337–F344 (2001).
R. Kozyraki. Cubilin, a multifunctional epithelial receptor: an overview. J. Mol. Med. 79:161–167 (2001).
S. M. Hammad, J. L. Barth, C. Knaak, and W. S. Argraves. Megalin acts in concert with cubilin mediate endocytosis of high density lipoproteins. J. Biol. Chem. 275:12003–12008 (2000).
I. Kolleck, H. Wissel, F. Guthmann, M. Schlame, P. Sinha, and B. Rustow. HDL-holoparticle uptake by alveolar type II cells: effect of vitamin E status. Am. J. Respir. Cell Mol. Biol. 27:57–63 (2002).
Acknowledgements
We thank Prof. Hidetoshi Tahara (Graduate School of Biomedical Sciences, Hiroshima University) for phase-contrast microscopic observation of the epithelial cells. We also thank the Institute of Laboratory Animal Science, the Natural Science Center for Basic Research and Development, and the Research Center for Molecular Medicine, Faculty of Medicine, Hiroshima University for the use of their facilities. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture in Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ikehata, M., Yumoto, R., Nakamura, K. et al. Comparison of Albumin Uptake in Rat Alveolar Type II and Type I-like Epithelial Cells in Primary Culture. Pharm Res 25, 913–922 (2008). https://doi.org/10.1007/s11095-007-9426-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9426-x