Abstract
Purpose
To develop a statistical model for predicting effect of food on the extent of absorption (area under the curve of time–plasma concentration profile, AUC) of drugs based on physicochemical properties.
Materials and Methods
Logistic regression was applied to establish the relationship between the effect of food (positive, negative or no effect) on AUC of 92 entries and physicochemical parameters, including clinical doses used in the food effect study, solubility (pH 7), dose number (dose/solubility at pH 7), calculated Log D (pH 7), polar surface area, total surface area, percent polar surface area, number of hydrogen bond donor, number of hydrogen bond acceptors, and maximum absorbable dose (MAD).
Results
For compounds with MAD ≥ clinical dose, the food effect can be predicted from the dose number category and Log D category, while for compounds with MAD < clinical dose, the food effect can be predicted from the dose number category alone. With cross validation, 74 out of 92 entries (80%) were predicted into the correct category. The correct predictions were 97, 79 and 68% for compounds with positive, negative and no food effect, respectively.
Conclusions
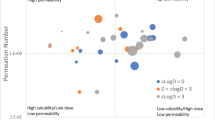
A logistic regression model based on dose, solubility, and permeability of compounds is developed to predict the food effect on AUC. Statistically, solubilization effect of food primarily accounted for the positive food effect on absorption while interference of food with absorption caused negative effect on absorption of compounds that are highly hydrophilic and probably with narrow window of absorption.
Similar content being viewed by others
References
S. D. Patil, L. Y. Ngo, P. Glue, and J. D. Unadkat. Intestinal absorption of ribavirin is preferentially mediated by the Na+-nucleoside purine (N1) transporter. Pharm. Res. 15:950–952 (1998).
H. Liedholmand and A. Melander. Concomitant food intake can increase the bioavailability of propranolol by transient inhibition of its presystemic primary conjugation. Clin. Pharmacol. Ther. 40:29–36 (1986).
J. J. Leyden. Absorption of minocycline and tetracycline: effect of food, milk and iron. Int. Congr. Symp. Ser.—R. Soc. Med. 95:87–92 (1985).
D. Brownand and R. Juhl. Decreased bioavailability of digoxacin due to antacids and kaolin-pectin. N. Engl. J. Med. 19:1034–1037 (1976).
J. Dressman. Comparison of canine and human gastrointestinal physiology. Pharm. Res. 3:123–131 (1986).
T. Kararli. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of human and commonly used laboratory animals. Biopharm. Drug Dispos. 16:351–380 (1995).
D. Fleisher, C. Li, Y. Zhou, L.-H. Pao, and A. Karim. Drug, meal and formulation interactions influencing drug absorption after oral administration: clinical implications. Clin. Pharmacokinet. 36:233–254 (1999).
L. Schmidtand and K. Dalhoff. Food-drug interactions. Drugs 62:1481–1502 (2002).
B. Singh. Effects of food on clinical pharmacokinetics. Clin. Pharmacokinet. 37:213–255 (1999).
Physician Desk Reference Electronic Library, http://www.thomsonhc.com/pdrel/librarian/ND_PR/Pdr Accessed 11/2004 – 02/2006.
ACD/PhysChem Batch, version 9.0. Advanced Chemistry Development, Inc. Toronto, Canada.
The United States Pharmacopeia. Authority of the United States Pharmacopeial Convention, 25th ed. National Publishing, Philadelphia, PA 2004.
Analytical Profiles of Drug Substances. Series editor: K. Florey. Academic, New York (1972–1991).
The Merck Index, 13th ed. Merck Research Laboratories, Rahway, NJ (2001).
USP DI Volume III, Approved Drug Products and Legal Requirements, 26th ed. United States Pharmacopeial Convention, Inc. Rockville, MD (2006).
D. Oh, R. Curl, and G. Amidon. Estimating the fraction dose absorbed from suspensions of poorly soluble compounds in humans: a mathematical model. Pharm. Res. 10:264–270 (1993).
W. Curatolo. Physical chemical properties of oral drug candidates in the discovery and exploratory development settings. Pharm. Sci. Technol. Today 1:387–393 (1998).
D. Sun, L. X. Yu, M. A. Hussain, D. A. Wall, R. L. Smith, and G. L. Amidon. In vitro testing of drug absorption for drug ‘developability’ assessment: forming an interface between in vitro preclinical data and clinical outcome. Curr. Opin. Drug Discov. Dev. 7:75–85 (2004).
SAS/STAT® User’s Guide, Version 6, Fourth Edition, Volume 2. Chapter 27, The LOGISTIC procedure, 1071–1126 (1990).
Y. Li. Mechanisms of region-dependent absorption of a weakly basic hiv protease inhibitor, indinavir: clinical ramifications and comparison with nelfinavir, pH. D thesis, University of Michigan, Ann Arbor, MI (2001).
N. Petri, C. Tannergren, D. Rungstad, and H. Lennernaes. Transport characteristics of fexofenadine in the Caco-2 Cell Model. Pharm. Res. 21:1398–1404 (2004).
S. Renand and E. J. Lien. Caco-2 cell permeability vs. human gastro-intestinal absorption: QSAR analysis. Prog. Drug Res. 54:1–23 (2000).
N. A. Kasim, M. Whitehouse, C. Ramachandran, M. Bermejo, H. Lennernas, A. S. Hussain, H. E. Junginger, S. A. Stavchansky, K. K. Midha, V. P. Shah, and G. L. Amidon. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Molecular Pharmaceutics 1:85–96 (2004).
A. J. Humberstone, C. J. H. Porter, and N. W. Charman. A physicochemical basis for the effect of food on the absolute oral bioavailability of halofantrine. J. Pharm. Sci. 85:525–529 (1996).
C.-Y. Wu and L. Z. Benet. Predicting drug disposition via application of BCS: transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm. Res. 22:11–23 (2005).
J. Fraser and P. Gibson. Mechanisms by which food intake elevates circulating levels of hyaluronan in humans. J. Intern. Med. 258:460–466 (2005).
G. L. Amidon, H. Lennernaes, V. Shah, and J. R. Crison. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 12:413–420 (1995).
E. M. Persson, A.-S. Gustafsson, A. S. Carlsson, R. G. Nilsson, L. Knutson, P. Forsell, G. Hanisch, H. Lennernaes, and B. Abrahamsson. The effects of food on the dissolution of poorly soluble drugs in human and in model small intestinal fluids. Pharm. Res. 22:2141–2151 (2005).
N. Ahuja, A. Singh, and B. Singh. Rofecoxib: an update on physicochemical, pharmaceutical, pharmacodynamic and pharmacokinetic aspects. J. Pharm. Pharmacol. 55:859–894 (2003).
E. Lipka, J. M. Hilfinger, C. A. Siersma, Y. Tsume, J. R. Crison, R. E. Ridgewell, and G. L. Amidon. Evaluation of Imiquimod and analogs with respect to their oral delivery potential. Proc. Int. Symp. Control. Release Bioact. Mater. 24:337–338 (1997).
I. D. Cockshott, S. D. Oliver, J. J. Young, K. J. Cooper, and D. C. Jones. The effect of food on the pharmacokinetics of the bicalutamide (‘Casodex’) enantiomers. Biopharm. Drug Dispos. 18:499–507 (1997).
W. N. Charman, M. C. Rogge, A. W. Boddy, and B. M. Berger. Effect of food and a monoglyceride emulsion formulation on danazol bioavailability. J. Clin. Pharmacol. 33:381–386 (1993).
A. Van Peer, R. Woestenborghs, J. Heykants, R. Gasparini, and G. Gauwenbergh. The effects of food and dose on the oral systemic availability of itraconazole in healthy subjects. Eur. J. Clin. Pharmacol. 36:423–426 (1989).
E. Liang, J. Proudfoot, and M. Yazdanian. Mechanisms of transport and structure-permeability relationship of sulfasalazine and its analogs in Caco-2 cell monolayers. Pharm. Res. 17:1168–1174 (2000).
C. Masungi, C. Borremans, B. Willems, J. Mensch, A. van Dijck, P. Augustijns, M. E. Brewster, and M. Noppe. Usefulness of a novel Caco-2 cell perfusion system. I. In vitro prediction of the absorption potential of passively diffused compounds. J. Pharm. Sci. 93:2507–2521 (2004).
E. K. Hussey, K. H. Donn, J. R. Powell, A. P. Lahey, and G. E. Pakes. Albuterol extended-release products: effect of food on the pharmacokinetics of single oral doses of Volmax and Proventil Repetabs in healthy male volunteers. J. Clin. Pharmacol. 31:561–4 (1991).
A. Tronde, B. Norden, H. Marchner, A.-K. Wendel, H. Lennernaes, and U. H. Bengtsson. Pulmonary absorption rate and bioavailability of drugs in vivo in rats: Structure-absorption relationships and physicochemical profiling of inhaled drugs. J. Pharm. Sci. 92:1216–1233 (2003).
J. Fuji, N. Inotsume, and M. Nakano. Effect of food on the bioavailability of bromazepam following oral administration in healthy volunteers. J. Pharmacobio-Dyn. 13:269–271 (1990).
Z. Kopitar, B. Vrhovac, L. Povsic, F. Plavsic, I. Francetic, and J. Urbancic. The effect of food and metoclopramide on the pharmacokinetics and side effects of bromocriptine. Eur. J. Drug Metab. Pharmacokinet. 16:177–181 (1991).
Y. M. Ponce, M. A. C. Perez, V. R. Zaldivar, M. B. Sanz, D. S. Mota, and F. Torrens. Prediction of intestinal epithelial transport of drug in (Caco-2) cell culture from molecular structure using in silico approaches during early drug discovery. Internet Electronic Journal of Molecular Design 4:124–150 (2005).
B. Reigner, J. Verweij, L. Dirix, J. Cassidy, C. Twelves, D. Allman, E. Weidekamm, B. Roos, L. Banken, M. Utoh, and B. Osterwalder. Effect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patients. Clin. Cancer Res. (an official journal of the American Association for Cancer Research) 4:941–948 (1998).
Bristol-Myers Squibb Internal Database. Bristol-Myers Squibb Co, New York, NY. Accessed 02/2006.
S. Yamashita, E. Hattori, A. Shimada, Y. Endoh, Y. Yamazaki, M. Kataoka, T. Sakane, and H. Sezaki. New methods to evaluate intestinal drug absorption mediated by oligopeptide transporter from in vitro study using Caco-2 cells. Drug Metab. Pharmacokinet. 17:408–415 (2002).
R. M. Menon and W. H. Barr. Comparison of ceftibuten transport across Caco-2 cells and rat jejunum mounted on modified ussing chambers. Biopharm. Drug Dispos. 24:299–308 (2003).
P. V. Desmond, P. J. Harman, N. Gannoulis, M. Kamm, and M. L. Mashford. The effect of an antacid and food on the absorption of cimetidine and ranitidine. J. Pharm. Pharmacol. 42:352–354 (1990).
A. Avdeef, P. Artursson, S. Neuhoff, L. Lazorova, J. Grasjoe, and S. Tavelin. Caco-2 permeability of weakly basic drugs predicted with the Double-Sink PAMPA pKfluxa method. Eur. J. Pharm. Sci. 24:333–349 (2005).
A. Shah, M.-C. Liu, D. Vaughan, and A. H. Heller. Oral bioequivalence of three ciprofloxacin formulations following single-dose administration: 500 mg tablet compared with 500 mg/10 mL or 500 mg/5 mL suspension and the effect of food on the absorption of ciprofloxacin oral suspension. J. Antimicrob. Chemother. 43:49–54 (1999).
N. M. Griffiths, B. H. Hirst, and N. L. Simmons. Active intestinal secretion of the fluoroquinolone antibacterials ciprofloxacin, norfloxacin and pefloxacin; a common secretory pathway? J. Pharmacol. Exp. Ther. 269:496–502 (1994).
K. Laitinen, A. Patronen, P. Harju, E. Loyttyniemi, L. Pylkkanen, T. Kleimola, and K. Perttunen. Timing of food intake has a marked effect on the bioavailability of clodronate. Bone (New York) 27:293–296 (2000).
J. Raiman, S. Tormalehto, K. Yritys, H. E. Junginger, and J. Monkkonen. Effects of various absorption enhancers on transport of clodronate through Caco-2 cells. Int. J. Pharm. 261:129–136 (2003).
C. Lippert, A. Keung, T. Arumugham, M. Eller, W. Hahne, and S. Weir. The effect of food on the bioavailability of dolasetron mesylate tablets. Biopharm. Drug Dispos. 19:17–19 (1998).
J. Dow, G. F. Di Francesco, and C. Berg. Comparison of the pharmacokinetics of dolasetron and its major active metabolite, reduced dolasetron, in dog. J. Pharm. Sci. 85:685–689 (1996).
J. J. Hanyok. Clinical pharmacokinetics of sotalol. Am. J. Cardiol. 72:19A–26A (1993).
T. D. Bjornsson, W. M. Troetel, and B. P. Imbimbo. Effect of food on the absorption of eptastigmine. Eur. J. Clin. Pharmacol. 54:243–247 (1998).
M. Stoltz, T. Arumugham, C. Lippert, D. Yu, V. Bhargava, M. Eller, and S. Weir. Effect of food on the bioavailability of fexofenadine hydrochloride (MDL 16 455A). Biopharm. Drug Dispos. 18:645–648 (1997).
F. Ingels, B. Beck, M. Oth, and P. Augustijns. Effect of simulated intestinal fluid on drug permeability estimation across Caco-2 monolayers. Int. J. Pharm. 274:221–232 (2004).
T. Shibuta, N. Inotsume, R. Iwaoku, and M. Nakano. Influence of food on pharmacokinetics and pharmacodynamics of furosemide. Byoin Yakugaku 14:12–16 (1988).
H. A. Semple, Y. K. Tam, and R. T. Coutts. Hydralazine pharmacokinetics and interaction with food: an evaluation of the dog as an animal model. Pharm. Res. 7:274–279 (1990).
L. X. Yu, A. B. Straughn, P. J. Faustino, Y. Yang, A. Parekh, A. B. Ciavarella, E. Asafu-Adjaye, M. U. Mehta, D. P. Conner, L. J. Lesko, and A. S. Hussain. The effect of food on the relative bioavailability of rapidly dissolving immediate-release solid oral products containing highly soluble drugs. Molecular Pharmaceutics 1:357–362 (2004).
T. Kosoglou, D. J. Kazierad, J. J. Schentag, J. E. Patrick, L. Heimark, E. Radwanski, D. Christopher, B. E. Flannery, and M. B. Affrime. Effect of food on the oral bioavailability of isosorbide-5-mononitrate administered as an extended-release tablet. J. Clin. Pharmacol. 35:151–158 (1995).
K. H. P. Moore, S. Shaw, A. L. Laurent, P. Lloyd, B. Duncan, D. M. Morris, M. J. O’Mara, and G. E. Pakes. Lamivudine/zidovudine as a combined formulation tablet: bioequivalence compared with lamivudine and zidovudine administered concurrently and the effect of food on absorption. J. Clin. Pharmacol. 39:593–605 (1999).
W. D. Hooper, R. G. Dickinson, and M. J. Eadie. Effect of food on absorption of lomefloxacin. Antimicrob. Agents Chemother. 34:1797–1799 (1990).
D. A. Volpe. Permeability classification of representative fluoroquinolones by a cell culture method. AAPS PharmSci 6: e13 (2004).
U. Busch, G. Heinzel, and H. Narjes. Effect of food on pharmacokinetics of meloxicam, a new nonsteroidal anti-inflammatory drug (NSAID). Agents Actions 32:52–53 (1991).
G. Ranaldi, K. Islam, and Y. Sambuy. Epithelial cells in culture as a model for the intestinal transport of antimicrobial agents. Antimicrob. Agents Chemother. 36:1374–1381 (1992).
N. B. Modi, B. Wang, W. T. Hu, and S. K. Gupta. Effect of food on the pharmacokinetics of osmotic controlled-release methylphenidate HCl in healthy subjects. Biopharm. Drug Dispos. 21:23–31 (2000).
J. Bass, K. V. Shepard, J. W. Lee, and J. Hulse. An evaluation of the effect of food on the oral bioavailability of sustained-release morphine sulfate tablets (ORAMORPH SR) after multiple doses. J. Clin. Pharmacol. 32:1003–1007 (1992).
J. Lettieri, R. Vargas, V. Agarwal, and P. Liu. Effect of food on the pharmacokinetics of a single oral dose of moxifloxacin 400 mg in healthy male volunteers. Clin. Pharmacokinet. 40:19–25 (2001).
M. N. Dudley, C. R. Marchbanks, S. C. Flor, and B. Beals. The effect of food or milk on the absorption kinetics of ofloxacin. Eur. J. Clin. Pharmacol. 41:569–571 (1991).
L. D’Angelo, F. De Ponti, F. Crema, M. Caravaggi, and A. Crema. Effect of food on the bioavailability of pidotimod in healthy volunteers. Arzneim.-Forsch. 44:1473–1475 (1994).
H. Y. Pan, A. R. DeVault, D. Brescia, D. A. Willard, M. E. McGovern, D. B. Whigan, and E. Ivashkiv. Effect of food on pravastatin pharmacokinetics and pharmacodynamics. Int. J. Clin. Pharmacol., Ther., Toxicol. 31:291–294 (1993).
M. A. H. Levine, S. E. Walker, and T. W. Paton. The effect of food and sucralfate on the bioavailability of S(+) and R(−) enantiomers of ibuprofen. J. Clin. Pharmacol. 32:1110–1114 (1992).
F. Faassen, G. Vogel, H. Spanings, and H. Vromans. Caco-2 permeability, P-glycoprotein transport ratios and brain penetration of heterocyclic drugs. Int. J. Pharm. 263:113–122 (2003).
L. I. Harrison, D. J. Riedel, K. E. Armstrong, M. B. Goldlust, and B. P. Ekholm. Effect of food on salsalate absorption. Ther. Drug Monit. 14:87–91 (1992).
G. R. Granneman and D. Mukherjee. The effect of food on the bioavailability of temafloxacin. A review of 3 studies. Clin. Pharmacokinet. 22:48–56 (1992).
D. R. Doose, S. A. Walker, L. G. Gisclon, and R. K. Nayak. Single-dose pharmacokinetics and effect of food on the bioavailability of topiramate, a novel antiepileptic drug. J. Clin. Pharmacol. 36:884–891 (1996).
D. Riendeau, M. D. Percival, C. Brideau, S. Charleson, D. Dube, D. Ethier, J. P. Falgueyret, R. W. Friesen, R. Gordon, G. Greig, J. Guay, J. Mancini, M. Ouellet, E. Wong, L. Xu, S. Boyce, D. Visco, Y. Girard, P. Prasit, R. Zamboni, I. W. Rodger, M. Gresser, A. W. Ford-Hutchinson, R. N. Young, and C. C. Chan. Etoricoxib (MK-0663): preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J. Pharmacol. Exp. Ther. 296:558–566 (2001).
M. Hashiguchi, H. Ogata, A. Maeda, Y. Hirashima, S. Ishii, Y. Mori, T. Amamoto, T. Handa, N. Otsuka, et al. No effect of high-protein food on the stereoselective bioavailability and pharmacokinetics of verapamil. J. Clin. Pharmacol. 36:1022–1028 (1996).
L. A. Nazareno, A. A. Holazo, R. Limjuco, S. Passe, S. K. Twardy, B. Min, and J. W. Massarella. The effect of food on pharmacokinetics of zalcitabine in HIV-positive patients. Pharm. Res. 12:1462–1465 (1995).
E. J. Seaber, R. W. Peck, D. A. Smith, J. Allanson, N. R. Hefting, J. J. Van Lier, F. A. E. Sollie, J. Wemer, and J. H. G. Jonkman. The absolute bioavailability and effect of food on the pharmacokinetics of zolmitriptan in healthy volunteers. Br. J. Clin. Pharmacol. 46:433–439 (1998).
J. D. Irvine, L. Takahashi, K. Lockhart, J. Cheong, J. W. Tolan, H. E. Selick, and J. R. Grove. MDCK (Madin-Darby canine kidney) cells: a tool for membrane permeability screening. J. Pharm. Sci. 88:28–33 (1999).
M. V. S. Varma, K. Sateesh, and R. Panchagnula. Functional role of P-glycoprotein in limiting intestinal absorption of drugs: contribution of passive permeability to P-glycoprotein mediated efflux transport. Molecular Pharmaceutics 2:12–21 (2005).
G. E. Chittick, C. Gillotin, J. A. McDowell, Y. Lou, K. D. Edwards, W. T. Prince, and D. S. Stein. Abacavir: absolute bioavailability, bioequivalence of three oral formulations, and effect of food. Pharmacotherapy 19:932–942 (1999).
G. Merino, A. I. Alvarez, J. G. Prieto, and R. B. Kim. The anthelmintic agent albendazole does not interact with P-glycoprotein. Drug Metab. Dispos. 30:365–369 (2002).
X. Meng, P. Mojaverian, M. Doedee, E. Lin, I. Weinryb, S. T. Chiang, and P. R. Kowey. Bioavailability of amiodarone tablets administered with and without food in healthy subjects. cAm. J. Cardiol. 87:432–435 (2001).
R. Dixon, A. L. Pozniak, H. M. Watt, P. Rolan, and J. Posner. Single-dose and steady-state pharmacokinetics of a novel microfluidized suspension of atovaquone in human immunodeficiency virus-seropositive patients. Antimicrob. Agents Chemother. 40:556–560 (1996).
H. Emori, S. Yokohama, and T. Nishihata. Small intestinal absorption of bropirimine in rats and effect of bile salt on the absorption. J. Pharm. Pharmacol. 47:487–492 (1995).
H. Emori, K. Yamamoto, S. Yokohama, and T. Nishihata. Bioavailability of bropirimine 250 mg tablet in dogs: effect of food. J. Pharm. Pharmacol. 47:822–826 (1995).
H. Saitoh, B. J. Aungst, M. Tohyama, Y. Hatakeyama, K. Ohwada, M. Kobayashi, H. Fujisaki, and K. Miyazaki. In vitro permeation of b-lactam antibiotics across rat jejunum and its correlation with oral bioavailability in humans. Br. J. Clin. Pharmacol. 54:445–448 (2002).
S. K. Paulson, M. B. Vaughn, S. M. Jessen, Y. Lawal, C. J. Gresk, B. Yan, T. J. Maziasz, C. S. Cook, and A. Karim. Pharmacokinetics of celecoxib after oral administration in dogs and humans: effect of food and site of absorption. J. Pharmacol. Exp. Ther. 297:638–645 (2001).
J. McEwen, G. Strauch, P. Perles, G. Pritchard, T. E. Moreland, J. Necciari, and J. P. Dickinson. Clopidogrel bioavailability: absence of influence of food or antacids. Semin. Thromb. Hemost. 25:47–50 (1999).
A. Nordqvist, J. Nilsson, T. Lindmark, A. Eriksson, P. Garberg, and M. Kihlen. A general model for prediction of Caco-2 cell permeability. QSAR & Combinatorial Science 23:303–310 (2004).
D. G. Blanchett, J. A. Green, A. Nara, R. Pospisil, R. C. Jarvis, R. J. Kasmer, D. A. Boyle, M. J. Cyronak, and C. N. Corder. The effect of food on pharmacokinetics and pharmacodynamics of fenoldopam in class III heart failure. Clin. Pharmacol. Ther. 49:449–456 (1991).
A. Clancy, J. Locke-Haydon, R. J. Cregeen, M. Ireson, and J. Ziemniak. Effect of concomitant food intake on absorption kinetics of fenoldopam (SK&F 82526) in healthy volunteers. Eur. J. Clin. Pharmacol. 32:103–106 (1987).
J. Lavelle, S. Follansbee, C. B. Trapnell, W. C. Buhles, K. G. Griffy, D. Jung, A. Dorr, and J. Connor. Effect of food on the relative bioavailability of oral ganciclovir. J. Clin. Pharmacol. 36:238–241 (1996).
K. A. Milton, G. Edwards, S. A. Ward, M. L. E. Orme, and A. M. Breckenridge. Pharmacokinetics of halofantrine in man: effects of food and dose size. Br. J. Clin. Pharmacol. 28:71–77 (1989).
R. L. Williams, J. Mordenti, R. A. Upton, E. T. Lin, W. L. Gee, C. D. Blume, and L. Z. Benet. Effects of formulation and food on the absorption of hydrochlorothiazide and triamterene or amiloride from combination diuretic products. Pharm. Res. 4:348–352 (1987).
I. Soria, P. Myhre, V. Horton, P. Ellefson, S. McCarville, K. Schmitt, and M. Owens. Effect of food on the pharmacokinetics and bioavailability of oral imiquimod relative to a subcutaneous dose. Int. J. Clin. Pharmacol. Ther. 38:476–481 (2000).
P. J. D. Kuang, C. Yeh, H. Haddix, M. Hesney, V. Hoagland, W. D. Ju, S. J. Justice, B. Osborne, A. T. Sterrett, J. A. Stone, E. Woolf, and S. Waldman. Single-dose pharmacokinetics of indinavir and the effect of food. Antimicrob. Agents Chemother. 42:1308 (1998).
N. N. Vachharajani, W. C. Shyu, D. S. Greene, and H. D. Uderman. Effects of food on the pharmacokinetics of irbesartan/hydrochlorothiazide combination tablet. Clin. Drug Investig. 16:399–404 (1998).
A. Avdeef, P. E. Nielsen, and O. Tsinman. PAMPA—a drug absorption in vitro model 11. Matching the in vivo unstirred water layer thickness by individual-well stirring in microtitre plates. Eur. J. Pharm. Sci. 22:365–374 (2004).
D. Rosillon, A. Stockis, G. Poli, D. Acerbi, R. Lins, and B. Jeanbaptiste. Food effect on the oral bioavailability of Manidipine: single dose, randomized, crossover study in healthy male subjects. Eur. J. Drug Metab. Pharmacokinet. 23:197–202 (1998).
C. Crevoisier, J. Handschin, J. Barre, D. Roumenov, and C. Kleinbloesem. Food increases the bioavailability of mefloquine. Eur. J. Clin. Pharmacol. 53:135–139 (1997).
A. Karim, L. F. Rozek, M. E. Smith, and K. G. Kowalski. Effects of food and antacid on oral absorption of misoprostol, a synthetic prostaglandin E1 analog. J. Clin. Pharmacol. 29:439–443 (1989).
D. S. Greene and R. H. Barbhaiya. Clinical pharmacokinetics of nefazodone. Clin. Pharmacokinet. 33: 260–275 (1997).
S. M. Abdel-Rahman and G. L. Kearns. Single-dose pharmacokinetics of a pleconaril (VP63843) oral solution and effect of food. Antimicrob. Agents Chemother. 42:2706–2709 (1998).
S. M. Abdel-Rahman and G. L. Kearns. Single oral dose escalation pharmacokinetics of pleconaril (VP 63843) capsules in adults. J. Clin. Pharmacol. 39:613–618 (1999).
B. J. Aungst, N. H. Nguyen, J. P. Bulgarelli, and K. Oates-Lenz. The influence of donor and reservoir additives on Caco-2 permeability and secretory transport of HIV protease inhibitors and other lipophilic compounds. Pharm. Res. 17:1175–1180 (2000).
J. Alsenz and E. Haenel. Development of a 7-day, 96-well Caco-2 permeability assay with high-throughput direct UV compound analysis. Pharm. Res. 20:1961–1969 (2003).
R. A. Ronfeld, K. D. Wilner, and B. A. Baris. Sertraline: chronopharmacokinetics and the effect of coadministration with food. Clin. Pharmacokinet. 32:50–55 (1997).
J. Shah, A. Fratis, D. Ellis, S. Murakami, and P. Teitelbaum. Effect of food and antacid on absorption of orally administered ticlopidine hydrochloride. J. Clin. Pharmacol. 30:733–736 (1990).
J. B. Lecaillon, J. Godbillon, J. Campestrini, C. Naquira, L. Miranda, R. Pacheco, R. Mull, and A. A. Poltera. Effect of food on the bioavailability of triclabendazole in patients with fascioliasis. Br. J. Clin. Pharmacol. 45:601–604 (1998).
B. A. Hamelin, S. Allard, L. Laplante, J. Miceli, K. D. Wilner, J. Tremblay, and M. LeBel. The effect of timing of a standard meal on the pharmacokinetics and pharmacodynamics of the novel atypical antipsychotic agent ziprasidone. Pharmacotherapy 18:9–15 (1998).
Acknowledgement
The authors would like to thank the following colleagues at Bristol-Myers Squibb for providing data and useful discussion: B. Vig, N. Mathias S. Lawrence, M. Fakes, S. Badawy, F. Zhao, S. Varia, M. Zheng, K. He, V. Rao.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s11095-007-9337-x
Rights and permissions
About this article
Cite this article
Gu, CH., Li, H., Levons, J. et al. Predicting Effect of Food on Extent of Drug Absorption Based on Physicochemical Properties. Pharm Res 24, 1118–1130 (2007). https://doi.org/10.1007/s11095-007-9236-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9236-1