Abstract
Purpose
The goal is to provide an overview on the advances in protection against chemotherapy-induced alopecia (CIA).
Materials and Methods
The four major parts of this review are (a) overview of the hair follicle biology, (b) characteristics of CIA, (c) state-of-the-art animal models of CIA, and (d) experimental approaches on protection against CIA.
Results
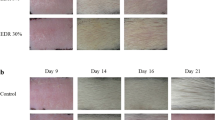
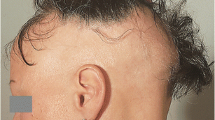
The hair follicle represents an unintended target of cancer chemotherapy. CIA is a significant side effect that compromises the quality of life of patients. Overcoming CIA represents an area of unmet needs, especially for females and children. Significant progresses have been made in the last decade on the pathobiology of CIA. The pharmacological agents under evaluation include drug-specific antibodies, hair growth cycle modifiers, cytokines and growth factors, antioxidants, cell cycle or proliferation modifiers, and inhibitors of apoptosis. Their potential applications and limitations are discussed.
Conclusion
Multiple classes of agents with different action mechanisms have been evaluated in animal CIA models. Most of these protective agents have activity limited to a single chemotherapeutic agent. In comparison, calcitriol and cyclosporine A have broader spectrum of activity and can prevent against CIA by multiple chemotherapeutic agents. Among the three agents that have been evaluated in humans, AS101 and Minoxidil were able to reduce the severity or shorten the duration of CIA but could not prevent CIA.


Similar content being viewed by others
Abbreviations
- Ara-C:
-
cytosine arabinoside
- CIA:
-
chemotherapy-induced alopecia
- EGF:
-
epidermal growth factor
- FGF:
-
fibroblast growth factor
- IRS:
-
inner root sheath
- ORS:
-
outer root sheath
- PTH:
-
parathyroid hormone
- PTHrP:
-
PTH-related protein
References
N. Carelle, E. Piotto, A. Bellanger, J. Germanaud, A. Thuillier, and D. Khayat. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer95:155–163 (2002).
V. J. Dorr. A practioner’s guide to cancer-related alopecia. Semin. Oncol.25:562–570 (1998).
C. Lindley, J. S. McCune, T. E. Thomason, D. Lauder, A. Sauls, S. Adkins, and W. T. Sawyer. Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract.7:59–65 (1999).
S. Pickard-Holley. The symptom experience of alopecia. Semin. Oncol. Nurs.11:235–238 (1995).
E. L. McGarvey, L. D. Baum, R. C. Pinkerton, and L. M. Rogers. Psychological sequelae and alopecia among women with cancer. Cancer Pract.9:283–289 (2001).
K. Munstedt, N. Manthey, S. Sachsse, and H. Vahrson. Changes in self-concept and body image during alopecia induced cancer chemotherapy. Support. Care Cancer5:139–143 (1997).
K. O. Baxley, L. K. Erdman, E. B. Henry, and B. J. Roof. Alopecia: effect on cancer patients’ body image. Cancer Nurs.7:499–503 (1984).
S. Harrison and R. Sinclair. Optimal management of hair loss (alopecia) in children. Am. J. Clin Dermatol.4:757–770 (2003).
L. Wagner and M. Gorely. Body image and patients experiencing alopecia as a result of cancer chemotherapy. Cancer Nurs.2:365–369 (1979).
D. Spiegel and J. Giese-Davis. Depression and cancer: mechanisms and disease progression. Biol. Psychiatry54:269–282 (2003).
T. Parker-Pope. Why curing your cancer may not be the best idea. Wall Street J., R1–R5, Dow Jones, 2003.
R. Paus, S. Muller-Rover, C. van Der Veen, M. Maurer, S. Eichmuller, G. Ling, U. Hofmann, K. Foitzik, L. Mecklenburg, and B. Handjiski. A comprehensive guide for the recognition and classification of distinct stages of hair follicle morphogenesis. J. Invest. Dermatol.113:523–532 (1999).
G. Cotsarelis, T. T. Sun, and R. M. Lavker. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell61:1329–1337 (1990).
R. M. Lavker, T. T. Sun, H. Oshima, Y. Barrandon, M. Akiyama, C. Ferraris, G. Chevalier, B. Favier, C. A. Jahoda, D. Dhouailly, A. A. Panteleyev, and A. M. Christiano. Hair follicle stem cells. J. Investig. Dermatol. Symp. Proc.8:28–38 (2003).
S. Lyle, M. Christofidou-Solomidou, Y. Liu, D. E. Elder, S. Albelda, and G. Cotsarelis. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell Sci.111(21):3179–3188 (1998).
R. J. Morris, Y. Liu, L. Marles, Z. Yang, C. Trempus, S. Li, J. S. Lin, J. A. Sawicki, and G. Cotsarelis. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol.22:411–417 (2004).
K. S. Stenn and R. Paus. Controls of hair follicle cycling. Physiol. Rev.81:449–494 (2001).
R. Paus, N. Krejci-Papa, L. Li, B. M. Czarnetzki, and R. M. Hoffman. Correlation of proteolytic activities of organ cultured intact mouse skin with defined hair cycle stages. J. Dermatol. Sci.7:202–209 (1994).
W. C. Weinberg, P. D. Brown, W. G. Stetler-Stevenson, and S. H. Yuspa. Growth factors specifically alter hair follicle cell proliferation and collagenolytic activity alone or in combination. Differentiation45:168–178 (1990).
S. Muller-Rover, E. J. Peters, V. A. Botchkarev, A. Panteleyev, and R. Paus. Distinct patterns of NCAM expression are associated with defined stages of murine hair follicle morphogenesis and regression. J. Histochem. Cytochem.46:1401–1410 (1998).
A. J. Reynolds and C. A. Jahoda. Hair follicle stem cells? A distinct germinative epidermal cell population is activated in vitro by the presence of hair dermal papilla cells. J. Cell Sci.99 (Pt 2):373–385 (1991).
E. A. Olsen. Disorders of Hair Growth: Diagnosis and Treatment. McGraw-Hill, Health Professions Division, 1994.
G. Cotsarelis. The hair follicle: dying for attention. Am. J. Pathol.151:1505–1509 (1997).
M. H. Hardy. The secret life of the hair follicle. Trends Genet.8:55–61 (1992).
H. Oshima, A. Rochat, C. Kedzia, K. Kobayashi, and Y. Barrandon. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell104:233–245 (2001).
M. Inaba, J. Anthony, and C. McKinstry. Histologic study of the regeneration of axillary hair after removal with subcutaneous tissue shaver. J. Invest. Dermatol.72:224–231 (1979).
R. F. Oliver. Whisker growth after removal of the dermal papilla and lengths of follicle in the hooded rat. J. Embryol. Exp. Morphol.15:331–347 (1966).
R. F. Oliver. Ectopic regeneration of whiskers in the hooded rat from implanted lengths of vibrissa follicle wall. J. Embryol. Exp. Morphol.17:27–34 (1967).
R. F. Oliver. The experimental induction of whisker growth in the hooded rat by implantation of dermal papillae. J. Embryol. Exp. Morphol.18:43–51 (1967).
C. Blanpain, W. E. Lowry, A. Geoghegan, L. Polak, and E. Fuchs. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell118: 635–648 (2004).
T. Tumbar, G. Guasch, V. Greco, C. Blanpain, W. E. Lowry, M. Rendl, and E. Fuchs. Defining the epithelial stem cell niche in skin. Science303:359–363 (2004).
L. Alonso and E. Fuchs. Stem cells in the skin: waste not, Wnt not. Genes Dev.17:1189–1200 (2003).
L. Alonso and E. Fuchs. Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. USA100 (Suppl 1):11830–11835 (2003).
H. B. Chase. Growth of the hair. Physiol. Rev.34:113–126 (1954).
G. Powis and K. L. Kooistra. Doxorubicin-induced hair loss in the Angora rabbit: a study of treatments to protect against the hair loss. Cancer Chemother. Pharmacol.20:291–296 (1987).
Bierman H.R., Kelly K.H., Knudson A.G., T. Maekawa, and G.M. Timmis. The influence of 1,4-dimethyl sulfonoxy-1,4-dimethylbutane (CB 2348, Dimethyl Myleran) in neoplastic disease. Ann. N.Y. Acad. Sci.68:1211–1222 (1958).
D. Batchelor. Hair and cancer chemotherapy: consequences and nursing care—a literature study. Eur. J. Cancer Care (Engl.)10:147–163 (2001).
A. M. Hussein. Chemotherapy-induced alopecia: new developments. South. Med. J.86:489–496 (1993).
B. D. Lawenda, H. M. Gagne, D. P. Gierga, A. Niemierko, W. M. Wong, N. J. Tarbell, G. T. Chen, F. H. Hochberg, and J. S. Loeffler. Permanent alopecia after cranial irradiation: dose–response relationship. Int. J. Radiat. Oncol. Biol. Phys.60: 879–887 (2004).
C. S. Wen, S. M. Lin, Y. Chen, J. C. Chen, Y. H. Wang, and S. H. Tseng. Radiation-induced temporary alopecia after embolization of cerebral arteriovenous malformations. Clin. Neurol. Neurosurg.105:215–217 (2003).
L. Li, L. B. Margolis, R. Paus, and R. M. Hoffman. Hair shaft elongation, follicle growth, and spontaneous regression in long-term, gelatin sponge-supported histoculture of human scalp skin. Proc. Natl. Acad. Sci. USA89:8764–8768 (1992).
L. Li, R. Paus, A. Slominski, and R. M. Hoffman. Skin histoculture assay for studying the hair cycle in vitro. Cell Dev. Biol.28A:695–698 (1992).
R. Paus, K. S. Stenn, and R. E. Link. Telogen skin contains an inhibitor of hair growth. Br. J. Dermatol.122:777–784 (1990).
R. Paus, B. Handjiski, S. Eichmuller et al. Chemotherapy-induced alopecia in mice-induction by cyclophosphamide, inhibition by cyclosporine-A, and modulation by dexamethasone. Am. J. Pathol.144:719–734 (1994).
D. Van Neste, B. De Brouwer, and M. Dumortier. Reduced linear hair growth rates of vellus and of terminal hairs produced by human balding scalp grafted onto nude mice. Ann. N. Y. Acad. Sci.642:480–482 (1991).
A. Domashenko, S. Gupta, and G. Cotsarelis. Efficient delivery of transgenes to human hair follicle progenitor cells using topical lipoplex. Nat. Biotechnol.18:420–423 (2000).
T. Hashimoto, T. Kazama, M. Ito, K. Urano, Y. Katakai, N. Yamaguchi, and Y. Ueyama. Histologic and cell kinetic studies of hair loss and subsequent recovery process of human scalp hair follicles grafted onto severe combined immunodeficient mice. J. Invest. Dermatol.115:200–206 (2000).
J. P. Sundberg and L. E. King, Jr. Mouse models for the study of human hair loss. Dermatol. Clin.14:619–632 (1996).
S. M. Jankovic and S. V. Jankovic. The control of hair growth. Dermatol. OnLine J.4:2 (1998).
A. M. Hussein, J. J. Jimenez, C. A. McCall, and A. A. Yunis. Protection from chemotherapy-induced alopecia in a rat model. Science249:1564–1566 (1990).
R. Cece, S. Cazzaniga, D. Morellie, L. Sfondrini, M. Bignotto, S. Menard, M. I. Colnaghi, and A. Balsari. Apoptosis of hair follicle cells during doxorubicin-induced alopecia in rats. Lab. Invest.75:601–609 (1996).
A. L. Balsari, D. Morelli, S. Menard, U. Veronesi, and M. I. Colnaghi. Protection against doxorubicin-induced alopecia in rats by liposome-entrapped monoclonal antibodies. FASEB J.8:226–230 (1994).
A. M. Hussein. Protection against cytosine arabinoside-induced alopecia by minoxidil in a rat animal model. Int. J. Dermatol.34:470–473 (1995).
J. J. Jimenez and A. A. Yunis. Protection from 1-beta-D-arabinofuranosylcytosine-induced alopecia by epidermal growth-factor and fibroblast growth-factor inthe rat model. Cancer Res.52:413–415 (1992).
M. B. Schilli, R. Paus, and A. Menrad. Reduction of intrafollicular apoptosis in chemotherapy-induced alopecia by topical calcitriol-analogs. J. Invest. Dermatol. 111:598–604 (1998).
D. J. Tobin, E. Hagen, V. A. Botchkarev, and R. Paus. Do hair bulb melanocytes undergo apoptosis during hair follicle regression (catagen)? J. Invest Dermatol.111:941–947 (1998).
G. Lindner, V. A. Botchkarev, N. V. Botchkareva, G. Ling, C. van Der Veen, and R. Paus. Analysis of apoptosis during hair follicle regression (catagen). Am. J. Pathol. 151:1601–1617 (1997).
A. A. Sharov, G. Z. Li, T. N. Palkina, T. Y. Sharova, B. A. Gilchrest, and V. A. Botchkarev. Fas and c-kit are involved in the control of hair follicle melanocyte apoptosis and migration in chemotherapy-induced hair loss. J. Invest. Dermatol.120:27–35 (2003).
U. Ohnemus, M. Unalan, B. Handjiski, and R. Paus. Topical estrogen accelerates hair regrowth in mice after chemotherapy-induced alopecia by favoring the dystrophic catagen response pathway to damage. J. Invest. Dermatol.122:7–13 (2004).
A. Shirai, H. Tsunoda, T. Tamaoki, and T. Kamiya. Topical application of cyclosporin A induces rapid-remodeling of damaged anagen hair follicles produced in cyclophosphamide administered mice. J. Dermatol. Sci.27:7–13 (2001).
J. M. Simister. Alopecia and cytotoxic drugs. Br. Med. J.2:1138 (1966).
P. Katsimbri, A. Bamias, and N. Pavlidis. Prevention of chemotherapy-induced alopecia using an effective scalp cooling system. Eur. J. Cancer36:766–771 (2000).
C. Protiere, K. Evans, J. Camerlo, M. P. D’Ingrado, G. Macquart-Moulin, P. Viens, D. Maraninchi, and D. Genre. Efficacy and tolerance of a scalp-cooling system for prevention of hair loss and the experience of breast cancer patients treated by adjuvant chemotherapy. Support. Care Cancer10:529–537 (2002).
I. G. Ron, Y. Kalmus, Z. Kalmus, M. Inbar, and S. Chaitchik. Scalp cooling in the prevention of alopecia in patients receiving depilating chemotherapy. Support. Care Cancer5:136–138 (1997).
G. Lutz. Effects of cyclosporin A on hair. Skin Pharmacol.7:101–104 (1994).
R. Paus, K. S. Stenn, and R. E. Link. The induction of anagen hair growth in telogen mouse skin by cyclosporine A administration. Lab. Invest.60:365–369 (1989).
M. Taylor, A. T. Ashcroft, and A. G. Messenger. Cyclosporin A prolongs human hair growth in vitro. J. Invest. Dermatol.100:237–239 (1993).
J. Liu, J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. Calcineurin is a common target of cyclophilin–cyclosporin A and FKBP–FK506 complexes. Cell66:807–815 (1991).
S. L. Schreiber. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell70:365–368 (1992).
A. M. Hussein, A. Stuart, and W. P. Peters. Protection against chemotherapy-induced alopecia by cyclosporin A in the newborn rat animal model. Dermatology190:192–196 (1995).
B. Sredni, R. H. Xu, M. Albeck, U. Gafter, R. Gal, A. Shani, T. Tichler, J. Shapira, I. Bruderman, R. Catane, B. Kaufman, J. K. Whisnant, K. L. Mettinger, and Y. Kalechman. The protective role of the immunomodulator AS101 against chemotherapy-induced alopecia studies on human and animal models. Int. J. Cancer65:97–103 (1996).
A. G. Messenger and J. Rundegren. Minoxidil: mechanisms of action on hair growth. Br. J. Dermatol.150:186–194 (2004).
M. Duvic, N. A. Lemak, V. Valero, S. R. Hymes, K. L. Farmer, G. N. Hortobagyi, R. J. Trancik, B. A. Bandstra, and L. D. Compton. A randomized trial of minoxidil in chemotherapy-induced alopecia. J. Am. Acad. Dermatol.35:74–78 (1996).
C. O. Granai, H. Frederickson, W. Gajewski, A. Goodman, A. Goldstein, and H. Baden. The use of minoxidil to attempt to prevent alopecia during chemotherapy for gynecologic malignancies. Eur. J. Gynaecol. Oncol.12:129–132 (1991).
R. Rodriguez, M. Machiavelli, B. Leone et al. Minoxidil (Mx) as a prophylaxis of doxorubicin-induced alopecia. Ann. Oncol.5:769–770 (1994).
D. Tran, R. D. Sinclair, A. P. Schwarer, and C. W. Chow. Permanent alopecia following chemotherapy and bone marrow transplantation. Aust. J. Dermatol.41:106–108 (2000).
D. M. Danilenko, B. D. Ring, and G. F. Pierce. Growth factors and cytokines in hair follicle development and cycling: recent insights from animal models and the potentials for clinical therapy. Mol. Med. Today2:460–467 (1996).
R. Paus and G. Cotsarelis. The biology of hair follicles. N. Engl. J. Med.341:491–497 (1999).
R. Imai, T. Jindo, K. Mochida, S. Shimaoka, K. Takamori, and H. Ogawa. Effects of cytokines, anti-cancer agents and cocarcinogen on DNA synthesis in hair bulb cells. J. Dermatol. Sci.5:73–80 (1993).
D. L. du Cros. Fibroblast growth factor and epidermal growth factor in hair development. J. Invest. Dermatol.101:106S–113S (1993).
D. L. du Cros. Fibroblast growth factor influences the development and cycling of murine hair follicles. Dev. Biol.156:444–453 (1993).
R. Halaban, R. Langdon, N. Birchall, C. Cuono, A. Baird, G. Scott, G. Moellmann, and J. McGuire. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J. Cell Biol.107:1611–1619 (1988).
C. Booth and C. S. Potten. Keratinocyte growth factor increases hair follicle survival following cytotoxic insult. J. Invest. Dermatol.114:667–673 (2000).
D. M. Danilenko, B. D. Ring, D. Yanagihara, W. Benson, B. Wiemann, C. O. Starnes, and G. F. Pierce. Keratinocyte growth factor is an important endogenous mediator of hair follicle growth, development, and differentiation. Normalization of the nu/nu follicular differentiation defect and amelioration of chemotherapy-induced alopecia. Am. J. Pathol. 147:145–154 (1995).
G. F. Pierce, D. Yanagihara, K. Klopchin, D. M. Danilenko, E. Hsu, W. C. Kenney, and C. F. Morris. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J. Exp. Med.179:831–840 (1994).
J. M. Hebert, T. Rosenquist, J. Gotz, and G. R. Martin. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell78:1017–1025 (1994).
T. A. Rosenquist and G. R. Martin. Fibroblast growth factor signalling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev. Dyn. 205:379–386 (1996).
F. D’Agostini, M. Bagnasco, D. Giunciuglio, A. Albini, and S. De Flora. Inhibition by oral N-acetylcysteine of doxorubicin-induced clastogenicity and alopecia, and prevention of primary tumors and lung micrometastases in mice. Int. J. Oncol.13:217–224 (1998).
J. J. Jimenez, H. S. Haung, and A. A. Yunis. Treatment with ImuVert/N-acetylcysteine protects rats from cyclophosphamide/ cytarabine-induced alopecia. Cancer Invest.10:271–276 (1992).
T. Kobayashi, K. Hashimoto, and K. Yoshikawa. Growth inhibition of human keratinocytes by 1,25-dihydroxyvitamin D3 is linked to dephosphorylation of retinoblastoma gene product. Biochem. Biophys. Res. Commun.196:487–493 (1993).
T. Kobayashi, H. Okumura, K. Hashimoto, H. Asada, S. Inui, and K. Yoshikawa. Synchronization of normal human keratinocyte in culture: its application to the analysis of 1,25-dihydroxyvitamin D3 effects on cell cycle. J. Dermatol. Sci.17:108–114 (1998).
S. E. Blutt, E. A. Allegretto, J. W. Pike, and N. L. Weigel. 1,25-dihydroxyvitamin D3 and 9-cis-retinoic acid act synergistically to inhibit the growth of LNCaP prostate cells and cause accumulation of cells in G1. Endocrinology138:1491–1497 (1997).
G. Hager, M. Formanek, C. Gedlicka, D. Thurnher, B. Knerer, and J. Kornfehl. 1,25(OH)2 vitamin D3 induces elevated expression of the cell cycle-regulating genes P21 and P27 in squamous carcinoma cell lines of the head and neck. Acta Oto-laryngol.121:103–109 (2001).
S. Kawa, K. Yoshizawa, M. Tokoo, H. Imai, H. Oguchi, K. Kiyosawa, T. Homma, T. Nikaido, and K. Furihata. Inhibitory effect of 220-oxa-1,25-dihydroxyvitamin D3 on the proliferation of pancreatic cancer cell lines. Gastroenterology110:1605–1613 (1996).
J. Kornfehl, M. Formanek, A. Temmel, B. Knerer, and M. Willheim. Antiproliferative effects of the biologically active metabolite of vitamin D3 (1,25 [OH]2 D3) on head and neck squamous cell carcinoma cell lines. Eur. Arch. Oto-rhino-laryngol.253:341–344 (1996).
M. Liu, M. H. Lee, M. Cohen, M. Bommakanti, and L. P. Freedman. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev.10:142–153 (1996).
J. J. Jimenez and A. A. Yunis. Vitamin D3 and chemotherapy-induced alopecia. Nutrition12:448–449 (1996).
J. J. Jimenez and A. A. Yunis. Protection from chemotherapy-induced alopecia by 1,25-dihydroxyvitamin D3. Cancer Res.52:5123–5125 (1992).
J. J. Jimenez, E. Alvarez, C. D. Bustamante, and A. A. Yunis. Pretreatment with 1,25(OH)2D3 protects from Cytoxan-induced alopecia without protecting the leukemic cells from Cytoxan. Am. J. Med. Sci.310:43–47 (1995).
R. Paus, M. B. Schilli, B. Handjiski, A. Menrad, B. M. Henz, and P. Plonka. Topical calcitriol enhances normal hair regrowth but does not prevent chemotherapy-induced alopecia in mice. Cancer Res.56:4438–4443 (1996).
Jimenez JJ, Beydoun M, and Yunis AA. 1,25(OH)2D3 protects from Taxol-induced alopecia. Clin. Res.42:128A (1994).
M. Hidalgo, D. Rinaldi, G. Medina, T. Griffin, J. Turner, and D.D. Von Hoff. A phase I trial of topical topitriol (calcitriol, 1,25-dihydroxyvitamin D-3) to prevent chemotherapy-induced alopecia. Anti-Cancer Drugs10:393–395 (1999).
M. F. Holick, S. Ray, T. C. Chen, X. Tian, and K. S. Persons. A parathyroid hormone antagonist stimulates epidermal proliferation and hair growth in mice. Proc. Natl. Acad. Sci. USA91:8014–8016 (1994).
E. M. Peters, K. Foitzik, R. Paus, S. Ray, and M. F. Holick. A new strategy for modulating chemotherapy-induced alopecia, using PTH/PTHrP receptor agonist and antagonist. J. Invest. Dermatol.117:173–178 (2001).
V. A. Botchkarev, E. A. Komarova, F. Siebenhaar, N.V. Botchkareva, P.G. Komarov, M. Maurer, B.A. Gilchrest, and A.V. Gudkov. p53 is essential for chemotherapy-induced hair loss. Cancer Res.60:5002–5006 (2000).
V. A. Botchkarev, E. A. Komarova, F. Siebenhaar, N. V. Botchkareva, A. A. Sharov, P. G. Komarov, M. Maurer, A. V. Gudkov, and B. A. Gilchrest. p53 Involvement in the control of murine hair follicle regression. Am. J. Pathol.158:1913–1919 (2001).
V. A. Botchkarev. Molecular mechanisms of chemotherapy-induced hair loss.J. Investig. Dermatol. Symp. Proc.8:72–75 (2003).
T. Tsuda, Y. Ohmori, H. Muramatsu, Y. Hosaka, K. Takiguchi, F. Saitoh, K. Kato, K. Nakayama, N. Nakamura, S. Nagata, and H. Mochizuki. Inhibitory effect of M50054, a novel inhibitor of apoptosis, on anti-Fas-antibody-induced hepatitis and chemotherapy-induced alopecia. Eur. J. Pharmacol.433:37–45 (2001).
Acknowledgment
This work is supported in part by a research grant R43CA107998 from the National Cancer Institute, DHHS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Lu, Z. & Au, J.LS. Protection Against Chemotherapy-Induced Alopecia. Pharm Res 23, 2505–2514 (2006). https://doi.org/10.1007/s11095-006-9105-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9105-3