Abstract
Purpose
The purpose of this study was to establish a primary culture of porcine lung epithelial cells as an alternative to the currently existing cell cultures from other species, such as e.g., rat or human. Primary porcine lung epithelial cells were isolated, cultivated and analyzed at distinct time points after isolation.
Materials and Methods
The main part of the work focused on the morphology of the cells and the detection of alveolar epithelial cell markers by using electron microscopy, immunofluorescence microscopy and immunoblotting. Regarding a later use for in vitro pulmonary drug absorption studies the barrier properties of the cell monolayer were evaluated by monitoring bioelectrical parameters and by marker transport.
Results
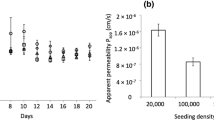
Epithelial cells isolated from porcine lung grew to confluent monolayers with typical intercellular junctions within a few days. Maximum transepithelial resistance of about 2,000 Ωcm2 was achieved and demonstrated the formation of a tight epithelial barrier. Permeability data of sodium fluorescein recommended a minimal transepithelial resistance of 600 Ωcm2 for transport studies. The cell population changed from a heterogeneous morphology and marker distribution (caveolin-1, pro-SP-C, surface sugars) towards a monolayer consisting of two cell types resembling type I and type II pneumocytes.
Conclusions
The porcine alveolar epithelial primary cell culture holds promise for drug transport studies, because it shares major hallmarks of the mammalian alveolar epithelium and it is easily available and scaled up for drug screening.









Similar content being viewed by others
Abbreviations
- (ab):
-
transport direction from apical to basolateral
- AP:
-
alkaline phosphatase
- ATCC:
-
american type culture collection
- Flu-Na:
-
sodium fluorescein
- hAEpC:
-
human alveolar epithelial cells
- kDa:
-
kilo dalton
- MPA:
-
maclura pomifera lectin
- pAEpC:
-
porcine alveolar epithelial cells
- pAEpC-n :
-
pAEpC isolation number n (cell batch)
- P app :
-
apparent permeability coefficient
- PD:
-
potential difference
- rAEpC:
-
rat alveolar epithelial cells
- RCA:
-
ricinus communis lectin
- rpm:
-
revolutions per minute
- SAGM:
-
small airway growth medium
- SDS:
-
sodium dodecyl sufate
- TEER:
-
transepithelial electrical resistance
- TEERmax :
-
maximum transepithelial electrical resistance
- TRIS:
-
Tris(hydroxymethyl)methylamine (buffer)
References
L. G. Dobbs. Isolation and culture of alveolar type II cells. Am. J. Physiol. 258(4 Pt 1):L134–L147 (1990).
K. J. Elbert, U. F. Schäfer, H. J. Schäfers, K. J. Kim, V. H. Lee, and C. M. Lehr. Monolayers of human alveolar epithelial cells in primary culture for pulmonary absorption and transport studies. Pharm. Res. 16(5):601–608, (1999).
C. Ehrhardt, K.-J. Kim, and C.-M. Lehr. Isolation and culture of human alveolar epithelial cells. In J. Picot, (ed.), Human Cell Culture Protocols, Humana, Totowa, New Jersey, 2005, pp. 207–216.
W. G. Pond and K. A. Houpt. The pig as a model in biomedical research. In Cornell University Press Ithaca (ed.), The Biology of the Pig, Comstock, London, 1978, pp. 13–64.
C. Hammer. Xenotransplantation—will it bring the solution to organ shortage? Ann. Transplant. 9(1):7–10 (2004).
N. Gardner, W. Haresign, R. Spiller, N. Farraj, J. Wiseman, H. Norbury, and L. Illum. Development and validation of a pig model for colon-specific drug delivery. J. Pharm. Pharmacol. 48:689–693 (1996).
O. Camber and P. Edman. Factors influencing the corneal permeability of prostaglandin F2alfa and its isopropyl ester in vitro. Int. J. Pharm. 37:27–32 (1987).
M. Fujii, S. Yamanouchi, N. Hori, N. Iwanaga, N. Kawaguchi, and M. Matsumoto. Evaluation of yucatan micropig skin for use as an in vitro model for skin permeation study. Biol. Pharm. Bull. 20(3):249–254 (1997).
M. B. Hansen, J. E. Thorboll, P. Christensen, N. Bindslev, and E. Skadhauge. Serotonin-induced short-circuit current in pig jejunum. Zentralbl. Veterinarmed. A. 41(2):110–120 (1994).
A. J. Hoogstraate, J. Coos Verhoef, A. Pijpers, L. A. van Leengoed, J. H. Verheijden, H. E. Junginger, and H. E. Bodde. In vivo buccal delivery of the peptide drug buserelin with glycodeoxycholate as an absorption enhancer in pigs. Pharm. Res. 13(8):1233–1237 (1996).
C. Wadell, E. Bjork, and O. Camber. Nasal drug delivery—evaluation of an in vitro model using porcine nasal mucosa. Eur. J. Pharm. Sci. 7(3):197–206 (1999).
S. Sangadala, P. Wallace, and J. Mendicino. Characterization of mucin glycoprotein-specific translation products from swine and human trachea, pancreas and colon. Mol. Cell. Biochem. 106(1):1–14 (1991).
M. Larsen and B. Rolin. Use of the Gottingen minipig as a model of diabetes, with special focus on type1 diabetes research. ILAR J. 45(3):303–313 (2004).
S. Fuchs, A. J. Hollins, M. Laue, U. F. Schaefer, K. Roemer, M. Gumbleton, and C.-M. Lehr. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell. Tissue Res. 311:31–45 (2003).
L. Bingle, T. B. Bull, B. Fox, A. Guz, R. J. Richards, and T. D. Tetley. Type II pneumocytes in mixed cell culture of human lung: a light and electron microscopic study. Environ. Health Perspect. 85:71–80 (1990).
A. C. Cunningham, D. S. Milne, J. Wilkes, J. H. Dark, T. D. Tetley, and J. A. Kirby. Constitutive expression of MHC and adhesion molecules by alveolar epithelial cells (type II pneumocytes) isolated from human lung and comparison with immunocytochemical findings. J. Cell. Sci. 107(Pt 2):443–449 (1994).
C. Gindorf, A. Steimer, C. M. Lehr, U. Bock, S. Schmitz, and E. Haltner. Markertransport über biologische Barrieren in vitro: Vergleich von Zellkulturmodellen für die Dünndarmschleimhaut, die Blut-Hirn Schranke und das Alveolarepithel der Lunge. Altex. 18(3):155–164 (2001).
LGC Promochem Offices (ATCC homepage). Cell lines and Hybridomas, Product Description. http://www.lgcpromochem-atcc.com/common/catalog/CellBiology/CellBiologyIndex.cfm (accessed 12/04/05), part of ATCC homepage. http://www. lgcpromochem-atcc.com (accessed 12/04/05).
C. Jumarie and C. Malo. Caco-2 cells cultured in serum-free medium as a model for the study of enterocytic differentiation in vitro. J. Cell. Physiol. 149(1):24–33 (1991).
H. Franke, H.-J. Galla, and C. T. Beuckmann. Primary cultures of brain microvessel endothelial cells: a valid and flexible model to study drug transport through the blood–brain barrier. Brain Res. Brain Res. Prot. 5:248–256 (2000).
J. H. Luft. Improvements in epoxy resin embedding methods. J. Biophys. Biochem. Cytol. 9(Feb):409–414 (1961).
M. Laue, G. Kiefer, B. Leis, N. Pütz, and P. Mestres. Environmental scanning electron microscopy of resin block face. Eur. Microsc. Anal. 97:13–15 (2005).
Roche Molecular Biochemicals. Lab FAQS: find a quick solution. Urs W. Hoffmann-Rohrer (DFKZ Heidelberg) (ed.), Mannheim, 2000.
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall. Protein measurement with the folin phenol reagent. J. Biol. Chem. Baltimore. 193:265–275 (1951).
U. K. Laemmli. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227(259):680–685 (1970).
L. G. Dobbs, M. C. Williams, and A. E. Brandt. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim. Biophys. Acta. 846(1):155–166 (1985).
G. R. Newman, L. Campbell, C. von Ruhland, B. Jasani, and M. Gumbleton. Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium: implications for alveolar epithelial type I cell function. Cell. Tissue Res. 295(1):111–120 (1999).
J. L. Alcorn, M. E. Smith, J. F. Smith, L. R. Margraf, and C. R. Mendelson. Primary cell culture of human type II pneumonocytes: maintenance of a differentiated phenotype and transfection with recombinant adenoviruses. Am. J. Respir. Cell. Mol. Biol. 17 (6):672–682 (1997).
Loxo Ltd. Product catalogue: LRP antibody. Novocastra Laboratories Ltd: p. 126. (2001).
J. D. Edelson, J. M. Shannon, and R. J. Mason. Alkaline phosphatase: a marker of alveolar type II cell differentiation. Am. Rev. Resp. Dis. 138(5):1268–1275 (1988).
A. Steimer, E. Haltner, and C.-M. Lehr. Cell culture models of the respiratory tract relevant to pulmonary drug delivery. J. Aerosol. Med. 18(2):137–182 (2005).
E. R. Weibel. Morphometry of the Human Lung. Academic, New York, 1963.
E. R. Weibel, P. Gehr, D. Haies, J. Gil, and M. Bachofen. The cell population of the normal lung. In A. Bouhhuys (ed.), Lung Cells in Disease, Amsterdam, North-Holland, 1976, pp. 3–16.
J. D. Crapo, B. E. Barry, P. Gehr, M. Bachofen, and E. R. Weibel. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 126(2):332–337 (1982).
L. G. Dobbs, M. C. Williams, and R. Gonzalez. Monoclonal antibodies specific to apical surfaces of rat alveolar type I cells bind to surfaces of cultured, but not freshly isolated, type II cells. Biochim. Biophys. Acta. 970(2):146–156 (1988).
D. M. Haies, J. Gil, and E. R. Weibel. Morphometric study of rat lung cells. I. Numerical and dimensional characteristics of parenchymal cell population. Am. Rev. Respir. Dis. 123(5):533–541 (1981).
J. M. Cheek, M. J. Evans, and E. D. Crandall. Type I cell-like morphology in tight alveolar epithelial monolayers. Exp. Cell Res. 184(2):375–387 (1989).
C. A. Diglio and Y. Kikkawa. The type II epithelial cells of the lung. IV. Adaption and behavior of isolated type II cells in culture. Lab. Invest. 37(6):622–631 (1977).
I. Y. Adamson and D. H. Bowden. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab. Invest. 30(1):35–42 (1974).
L. Campbell, A. J. Hollins, A. Al-Eid, G. R. Newman, C. von Ruhland, and M. Gumbleton. Caveolin-1 expression and caveolae biogenesis during cell transdifferentiation in lung alveolar epithelial primary cultures. Biochem. Biophys. Res. Commun. 262(3):744–751 (1999).
Y. Matsukawa, H. Yamahara, F. Yamashita, V. H. Lee, E. D. Crandall, and K. J. Kim. Rates of protein transport across rat alveolar epithelial cell monolayers. J. Drug Target. 7(5):335–342 (2000).
K. J. Kim, Z. Borok, and E. D. Crandall. A useful in vitro model for transport studies of alveolar epithelial barrier. Pharm. Res. 18(3):253–255 (2001).
P. Vodicka, K. J. Smetana, B. Dvorankova, T. Emerick, Y. Xu, J. Ourednik, V. Ourednik, and J. Motlik. The miniature pig as an animal model in biomedical research. Ann. NY Acad. Sci. 1049:161–171 (2005).
T. F. Allred, R. R. Mercer, R. F. Thomas, H. Deng, and R. L. Auten. Brief 95% O2 exposure effects on surfactant protein and mRNA in rat alveolar and bronchiolar epithelium. Am. J. Physiol. 276(6 Pt 1):L999–L1009 (1999).
G. F. Ross, M. Ikegami, W. Steinhilber, and A. H. Jobe. Surfactant protein C in fetal and ventilated preterm rabbit lungs. Am. J. Physiol. 277(6 Pt 1):L1104–L1108 (1999).
V. Wong, D. Ching, P. D. McCrea, and G. L. Firestone. Glucocorticoid down-regulation of fascin protein expression is required for the steroid-induced formation of tight junctions and cell–cell interactions in rat mammary epithelial tumor cells. J. Biol. Chem. 274(9):5443–5453 (1999).
H. Wan, H. L. Winton, C. Soeller, G. A. Stewart, P. J. Thompson, D. C. Gruenert, M. B. Cannell, D. R. Garrod, and C. Robinson. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o−. Eur. Respir. J. 15(6):1058–1068 (2000).
Y. Man, V. J. Hart, C. J. Ring, S. Sanjar, and M. R. West. Loss of epithelial integrity resulting from E-cadherin dysfunction predisposes airway epithelial cells to adenoviral infection. Am. J. Respir. Cell Mol. Biol. 23(5):610–617 (2000).
C. Ehrhardt, C. Kneuer, M. Laue, U. F. Schaefer, K. J. Kim, and C. M. Lehr. 16HBE14o− human bronchial epithelial cell layers express P-glycoprotein, lung resistance-related protein, and caveolin-1. Pharm. Res. 20(4):545–551 (2003).
Acknowledgments
We would like to thank Dr. Carsten Ehrhardt for helpful discussions and cooperation in immunostaining and confocal laser scanning microscopy, Birgit Leis and Norbert Pütz for technical assistance in electron microscopy and Manuel Birke (Symbiotec) for his kind introduction to the BioRad Scanner.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steimer, A., Laue, M., Franke, H. et al. Porcine Alveolar Epithelial Cells in Primary Culture: Morphological, Bioelectrical and Immunocytochemical Characterization. Pharm Res 23, 2078–2093 (2006). https://doi.org/10.1007/s11095-006-9057-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-9057-7