No Heading
Purpose.
To introduce partially linear mixed effects models (PLMEMs), to illustrate their use, and to compare the power and Type I error rate in detecting a covariate effect with nonlinear mixed effects modeling using NONMEM.
Methods.
Sparse concentration-time data from males and females (1:1) were simulated under a 1-compartment oral model where clearance was sex-dependent. All possible combinations of number of subjects (50, 75, 100, 150, 250), samples per subject (2, 4, 6), and clearance multipliers (1 to 1.25) were generated. Data were analyzed with and without sex as a covariate using PLMEM (maximum likelihood estimation) and NONMEM (first-order conditional estimation). Four covariate screening methods were examined: NONMEM using the likelihood ratio test (LRT), PLMEM using the LRT, PLMEM using Wald’s test, and analysis of variance (ANOVA) of the empirical Bayes estimates (EBEs) for CL treating sex as a categorical variable. The percent of simulations rejecting the null hypothesis of no covariate effect at the 0.05 level was determined. 300 simulations were done to calculate power curves and 1000 simulations were done (with no covariate effect) to calculate Type I error rate. Actual implementation of PLMEMs is illustrated using previously published teicoplanin data.
Results.
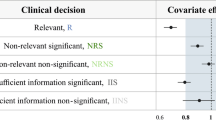
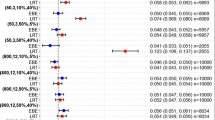
Type I error rates were similar between PLMEM and NONMEM using the LRT, but were inflated (as high as 36%) based on PLMEM using Wald’s test. Type I error rate tended to increase as the number of observations per subject increased for the LRT methods. Power curves were similar between the PLMEM and NONMEM LRT methods and were slightly more than the power curve using ANOVA on the EBEs of CL. 80% power was achieved with 4 samples per subject and 50 subjects total when the effect size was approximately 1.07, 1.07, 1.08, and 1.05 for LRT using PLMEMs, LRT using NONMEM, ANOVA on the EBEs, and Wald’s test using PLMEMs, respectively.
Conclusions.
PLMEM and NONMEM covariate screening using the LRT had similar Type I error rates and power under the data generating model. PLMEMs offers a viable alternative to NONMEM-based covariate screening.
Similar content being viewed by others
References
1. United States Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, and Center for Biologics Evaluation and Research. Guidance for Industry: Population Pharmacokinetics. 1999.
2. E. Gibiansky, S. Mallikaarjun, and S. L. Bramer. Nonparametric population pharmacokinetics of cilostazol. 1997. American Association of Pharmaceutical Scientists Annual Meeting, Boston, MA.
3. D. Ruppert, M. P. Wand, and R. J. Carroll. Semi Parametric Regression, Cambridge University Press, Cambridge, UK, 2003.
4. W. Hardle, H. Liang, and J. Gao. Partially Linear Models, Springer Verlag, Rockville, MD, 2001.
5. A. J. Boeckmann, L. B. Sheiner, and S. L. Beal. NONMEM User’s Guide, University of California, San Francisco, 1994.
6. J. Neter, M. H. Kutner, C. J. Nachtsheim, and W. Wasserman. Applied Linear Statistical Models, 4th ed., Irwin Press, Chicago, 1996.
7. J. Mandel. Use of the singular value decomposition in regression analysis. Am. Statistician 36:15–24 (1982).
8. K. Park, D. Verotta, and L. B. Sheiner. A semiparametric method for describing noisy population pharmacokinetic data. J. Pharmacokinet. Biopharm. 25:615–642 (1997).
9. G. Verbeke and G. Molenberghs. Linear Mixed Models in Practice: A SAS-Oriented Approach, Springer-Verlag, New York, 1997.
10. R. C. Littell, G. A. Milliken, W. W. Stroup, and R. D. Wolfinger. SAS Sytem for Mixed Models, SAS Institute, Cary, NC, 1996.
11. J. Pinheiro and D. M. Bates. Mixed-Effect Models in S and S-Plus, Springer-Verlag, New York, 2000.
12. Y. Wang. Smoothing spline models with correlated random errors. J. Am. Stat. Assoc. 93:341–348 (1998).
13. J. A. Steer, R. P. G. Papini, A. P. R. Wilson, S. Dhillon, M. F. Hichens, D. A. McGrouther, J. D. Frame, and N. Parkhouse. Pharmacokinetics of a single dose of teicoplanin in burn patients. J. Antimicrob. Chemother. 37:545–553 (1996).
14. F. Podczeck, S. Dhillon, and A. P. R. Wilson. The assessment of pharmacokinetic parameters of teicoplanin in burns comparing the methods of nonlinear curve fitting and quantified maximum entropy. Int. J. Pharmaceutics 142:235–246 (1996).
15. G. E. P. Box and D. R. Cox. An analysis of transformations. J. Royal Stat. Soc. B 26:211–243 (1964).
16. F. E. Satterthwaite. An approxite distribution of estimates of variance components. Biometrics Bull. 2:110–114 (1946).
17. E. I. Ette. Comparing non-hierarchical models: application to non-linear mixed effects modeling. Comp. Biol. Med. 6:505–512 (1996).
18. P. L. Bonate. A brief introduction to Monte Carlo simulation. Clin. Pharmacokin. 40:15–22 (2001).
19. D. Verotta. Building population pharmacokinetic-pharmacodynamic models using trees. In L. Balant and L. Aarons (eds.), The Population Approach: Measuring and Managing Variability in Response, Concentration, and Dose, Commission of the European Communities, European Cooperation in the Field of Scientific and Technical Research, Brussels, 1997.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonate, P. Covariate Detection in Population Pharmacokinetics Using Partially Linear Mixed Effects Models. Pharm Res 22, 541–549 (2005). https://doi.org/10.1007/s11095-005-2492-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-2492-z