Abstract
Multireference quantum chemical research with the aid of complete active space self-consistent field approach was performed to study the elementary reactions of \({{\text {CH}}_4}\) with \({\text {O}}_2\) in \({a^1\varDelta _g}\), \({b^1\varSigma _g^+}\), \({c^1\varSigma _u^-}\), and \({A^{\prime 3} \varDelta _u}\) electronically excited states highly relevant for plasma-assisted combustion and for plasma-chemical fuel reforming. The thermodynamically and kinetically favorable reaction pathways and likely intersystem crossings for the first step of the methane oxidation have been found out. The key energy values were refined based upon the extended multiconfiguration quasi-degenerate 2nd-order perturbation theory. It has been exhibited that the reaction of \({{\text {O}}_2(a^1\varDelta _g)}\) and \({{\text {O}}_2(A^{\prime 3} \varDelta _u)}\) with \({{\text {CH}}_4}\) proceeds through the abstraction of hydrogen with fairly low energy barriers that led to the formation of the \(\hbox {HO}_2\) molecule in \({^2A^{\prime \prime }}\) and \({^2A^{\prime }}\) electronic states, respectively. These results were compared with the findings of previous theoretical investigations. The oxygen molecule in singlet sigma b state was evinced to be nonreactive with regard to the methane. However, for \({c^1\varSigma _u^-}\) state, the reactive interaction was nevertheless found possible due to the significant probability of the nonadiabatic transitions. Appropriate thermal rate constants for revealed channels have been calculated employing variational transition-state theory and capture approximation. Corresponding three-parameter Arrhenius expressions for the broad temperature range (\(T=300\)–3000 K) were reported.






Similar content being viewed by others
References
Adamovich IV, Lempert WR (2015) Challenges in understanding and development of predictive models of plasma assisted combustion. Plasma Phys Control Fusion 57:014001. https://doi.org/10.1088/0741-3335/57/1/014001
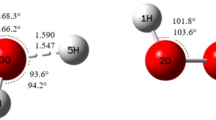
Andrienko GA. Chemcraft version 1.8. http://www.chemcraftprog.com
Aranda C, Richaud A, Mendez F, Dominguez A (2018) Theoretical rate constant of methane oxidation from the conventional transition-state theory. J Mol Model 24:294
Bao JL, Truhlar DG (2017) Variational transition state theory: theoretical framework and recent developments. Chem Soc Rev 46:7548–7596. https://doi.org/10.1039/c7cs00602k
Barone V (2005) Anharmonic vibrational properties by a fully automated second-order perturbative approach. J Chem Phys 122:014108
Baulch DL, Bowman CT, Cobos CJ, Cox RA, Just T, Kerr JA, Pilling MJ, Stocker D, Troe J, Tsang W, Walker RW, Warnatz J (2005) Evaluated kinetic data for combustion modeling. J Phys Chem Ref Data 34(3):757–1397. https://doi.org/10.1063/1.1748524
Becker KH, Groth W, Schurath U (1971) The quenching of metastable \({\text{O}}_2(^1\Delta _g)\) and \({\text{O}}_2(^1\Sigma _g^+)\) molecules. Chem Phys Lett 8:259–262
Blin-Simiand N, Jorand F, Magne L, Pasquiers S, Postel C, Vacher JR (2008) Plasma reactivity and plasma-surface interactions during treatment of toluene by a dielectric barrier discharge. Plasma Chem Plasma Process 28:429–466
Bodesheim M, Schmidt R (1997) Chemical reactivity of sigma singlet oxygen \({\text{O}}_2(b^1\Sigma _g^+)\). J Phys Chem A 101:5672–5677
Bross DH, Jasper AW, Ruscic B, Wagner AF (2019) Toward accurate high temperature anharmonic partition functions. Proc Combust Inst 37:315–322
Bruggeman PJ, Iza F, Brandenburg R (2017) Foundations of atmospheric pressure non-equilibrium plasmas. Plasma Sources Sci Technol 26:123,002
Carr RW (2007) Elements of chemical kinetics. In: Green NJB (ed) Comprehensive chemical kinetics, vol 42. Elsevier, Amsterdam, pp 43–99
Castela M, Fiorina B, Coussement A, Gicquel O, Darabiha N, Laux CO (2016) Modelling the impact of non-equilibrium discharges on reactive mixtures for simulations of plasma-assisted ignition in turbulent flows. Combust Flame 166:133–147. https://doi.org/10.1016/j.combustflame.2016.01.009
Chakraborty A, Truhlar DG, Bowman JM, Carter S (2004) Calculation of converged rovibrational energies and partition function for methane using vibrational-rotational configuration interaction. J Chem Phys 121:2071
Chen Q, Yang X, Sun J, Zhang X, Mao X, Ju Y, Koel BE (2017) Pyrolysis and oxidation of methane in a RF plasma reactor. Plasma Chem Plasma Process 37:1551–1571
Chukalovsky AA, Klopovsky KS, Palov AP, Mankelevich YA, Rakhimova TV (2016) Reaction of hydrogen atoms with singlet delta oxygen (\({\text{O}}_2(a^1\Delta _g)\)). Is everything completely clear? J Phys D Appl Phys 49:485202
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory. J Chem Phys 126:084108
Deng J, He L, Liu X, Chen Y (2018) Numerical simulation of plasma-assisted combustion of methane–air mixtures in combustion chamber. Plasma Sci Technol 20:125502. https://doi.org/10.1088/2058-6272/aacdef
Dunlea EJ, Talukdar RK, Ravishankara AR (2005) Kinetic studies of the reactions of \({\text{O}}_2(b^1\Sigma _g^+)\) with several atmospheric molecules. J Phys Chem A 109:3912–3920
Eckert Z, Tsolas N, Togai K, Chernukho A, Yetter RA, Adamovich IV (2018) Kinetics of plasma-assisted oxidation of highly diluted hydrocarbon mixtures excited by a repetitive nanosecond pulse discharge. J Phys D Appl Phys 51:374002
Fan X, McLaughlin JB, Melman A, Mededovic-Thagard S (2017) Quantum chemical approach for determining degradation pathways of phenol by electrical discharge plasmas. Plasma Chem Plasma Process 37:5–28
Fedorov DG (1999) Theoretical study of spin-orbit coupling in molecules. Retrospective theses and dissertations. Paper 12662, Iowa State University, Ames, Iowa
Fedorov DG, Koseki S, Schmidt MW, Gordon MS (2003) Spin–orbit coupling in molecules: chemistry beyond the adiabatic approximation. Int Rev Phys Chem 22(3):551–592
Fernandez-Ramos A, Miller JA, Klippenstein SJ, Truhlar DG (2006) Modeling the kinetics of bimolecular reactions. Chem Rev 106:4518–4584
Filimonova EA, Bocharov AN, Dobrovolskaya AS, Bityurin VA (2019) Influence of nanoseconds pulsed discharges on the composition of intermediate and final combustion products in the HCCI engine. Plasma Chem Plasma Process 39:683–694. https://doi.org/10.1007/s11090-019-09964-x
Fracchia F, Cimiraglia R, Angeli C (2015) Assessment of multireference perturbation methods for chemical reaction barrier heights. J Phys Chem A 119:5490–5495
Freidzon A, Tsybizova A (2017) CASSCF and Firefly: a tutorial. https://www.researchgate.net/publication/317106028. Accessed Jan 2019
Gimenez-Lopez J, Millera A, Bilbao R, Alzueta MU (2015) Experimental and kinetic modeling study of the oxy-fuel oxidation of natural gas, \({\text{CH}}_4\) and \(\text{ C }_2{\text{H}}_6\). Fuel 160:404–412
Gonzalez C, Schlegel BH (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2162
Granovsky AA. Firefly V. 8.2.0. http://classic.chem.msu.su/gran/firefly/index.html. Accessed Jan 2019
Granovsky AA (2011) Extended multi-configuration quasi-degenerate perturbation theory: the new approach to multi-state multi-reference perturbation theory. J Chem Phys 134:214113
Harding LB, Klippenstein SJ, Jasper AW (2007) Ab initio methods for reactive potential surfaces. Phys Chem Chem Phys 9:4055–4070
Harvey JN (2007) Understanding the kinetics of spin-forbidden chemical reactions. Phys Chem Chem Phys 9:331–343
Hashemi H, Christensen JM, Gersen S, Levinsky H, Klippenstein SJ, Glarborg P (2016) High-pressure oxidation of methane. Combust Flame 172:349–364
Helgaker T, Klopper W, Koch H, Noga J (1997) Basis-set convergence of correlated calculations on water. J Chem Phys 106:9639–9646
Herron JT, Green DS (2001) Chemical kinetics database and predictive schemes for nonthermal humid air plasma chemistry. Part II. Neutral species reactions. Plasma Chem Plasma Process 21(3):459–481
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure, constants of diatomic molecules, vol 4. Van Nostrand Reinhold, New York
Jacox ME (2003) Vibrational and electronic energy levels of polyatomic transient molecules. Supplement B. J Phys Chem Ref Data 32:1–437
Jasper AW, Klippenstein SJ, Harding LB (2009) Theoretical rate coefficients for the reaction of methyl radical with hydroperoxyl radical and for methylhydroperoxide decomposition. Proc Combust Inst 32:279–286. https://doi.org/10.1016/j.proci.2008.05.036
Jasper AW, Miller JA (2011) Theoretical unimolecular kinetics for \({\text{CH}}_4\)+M=\({\text{CH}}_3\)+H + M in eight baths, M = He, Ne, Ar, Kr, \({\text{H}}_2\), \({\text{N}}_2\), CO, and \({\text{CH}}_4\). J Phys Chem A 115:6438–6455
Ju Y, Lefkowitz JK, Reuter CB, Won SH, Yang X, Yang S, Sun W, Jiang Z, Chen Q (2016) Plasma assisted low temperature combustion. Plasma Chem Plasma Process 36:85–105
Ju Y, Sun W (2015) Plasma assisted combustion: dynamics and chemistry. Prog Energy Combust Sci 48:21–83
Kaledin AL, Heaven MC, Morokuma K (2001) Theoretical prediction of the rate constant for I + \({\text{O}}_2(a^1\Delta _g)\) electronic energy transfer: a surface-hopping trajectory study. J Chem Phys 114(1):215–224
Kendall RA, Dunning TH Jr, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96:6796–6806
Kendrick B, Pack RT (1995) Potential energy surfaces for the low-lying \(^2\)A″ and \(^2\)A′ states of \({\text{HO}}_2\): use of the diatomics in molecules model to fit ab initio data. J Chem Phys 102:1994–2012. https://doi.org/10.1063/1.468765
Khvatov NA, Zagidullin MV, Tolstov GI, Medvedkov IA, Mebel AM, Heaven MC, Azyazov VN (2019) Product channels of the reactions of \({\text{O}}_2(b^1\Sigma _g^+)\). Chem Phys 521:85–91. https://doi.org/10.1016/j.chemphys.2019.01.025
Konnov AA (2015) On the role of excited species in hydrogen combustion. Combust Flame 162:3755–3772. https://doi.org/10.1016/j.combustflame.2015.07.014
Koseki S, Schmidt MW, Gordon MS (1998) Effective nuclear charges for the first- through third-row transition metal elements in spin–orbit calculations. J Phys Chem A 102:10430–10435
Landau LD, Lifshitz EM (1977) Quantum mechanics: non-relativistic theory, vol 3. Pergamon Press, New York
Langhoff SR, Jaffe RL (1979) Theoretical study of the four lowest doublet electronic states of the hydroperoxyl radical: application to photodissociation. J Chem Phys 71:1475–1486
Lebedev AV, Deminsky MA, Zaitzevsky AV, Potapkin BV (2013) Effect of \({\text{O}}_2(a^1\Delta _g)\) on the low-temperature mechanism of \({\text{CH}}_4\) oxidation. Combust Flame 160:530–538. https://doi.org/10.1016/j.combustflame.2012.11.020
Lee DH, Kim KT, Song YH, Kang WS, Jo S (2013) Mapping plasma chemistry in hydrocarbon fuel processing processes. Plasma Chem Plasma Process 33:249–269
Lehman JH, Lester MI, Klos J, Alexander MH, Dagdigian PJ, Herraez-Aguilar D, Aoiz FJ, Brouard M, Chadwick H, Perkins T, Seamons SA (2013) Electronic quenching of OH \(A^2\Sigma ^{+}\) induced by collisions with Kr atoms. J Phys Chem A 117:13481–13490
Loukhovitski BI, Sharipov AS, Starik AM (2017) Quantum chemical study of small Al\(_n\)B\(_m\) clusters: structure and physical properties. Chem Phys 493:61–76
Lu X, Naidis GV, Laroussi M, Reuter S, Graves DB, Ostrikov K (2016) Reactive species in non-equilibrium atmospheric-pressure plasmas: generation, transport, and biological effects. Phys Rep 630:1–84
Mai TVT, v Duong M, Le XT, Huynh LK, Ratkiewicz A (2014) Direct ab initio dynamics calculations of thermal rate constants for the \({\text{CH}}_4\) + \({\text{O}}_2\) = \({\text{CH}}_3\) + \({\text{HO}}_2\) reaction. Struct Chem 25:1495–1503
Mao X, Rousso A, Chen Q, Ju Y (2019) Numerical modeling of ignition enhancement of \({\text{CH}}_4\)/\({\text{O}}_2\)/He mixtures using a hybrid repetitive nanosecond and DC discharge. Proc Combust Inst 37:5545–5552. https://doi.org/10.1016/j.proci.2018.05.106
Matsika S (2007) Chapter 2: Conical intersections in molecular systems. In: Lipkowitz KB, Cundari TR, Boyd DB (eds) Reviews in computational chemistry, vol 23. Wiley, Hoboken, pp 83–124
Matsunaga N, Koseki S (2004) Chapter 2: Modeling of spin-forbidden reactions. In: Lipkowitz KB, Larter R, Cundari TR, Boyd DB (eds) Reviews in computational chemistry, vol 20. Wiley, Hoboken, pp 101–152
Mayer SW, Schieler L (1968) Activation energies and rate constants computed for reactions of oxygen with hydrocarbons. J Phys Chem 72:2628–2631
Carr RW Jr (1972) Predictions of the rates of hydrogen abstraction by \({\text{CH}}_2\)(\(^3B_1\)) by the bond-energy bond-order method. J Phys Chem 76:1581–1586
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (1999) A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys 110:2822–2828
Mebel AM, Hayashi M, Kislov VV, Lin SH (2004) Theoretical study of oxygen isotope exchange and quenching in the O(\(^1D\)) + C\({\text{O}}_2\) reaction. J Phys Chem A 108:7983–7994
Mebel AM, Lin SH (1997) Excited electronic states of the methyl radical. Ab initio molecular orbital study of geometries, excitation energies and vibronic spectra. Chem Phys 215:329–341
Medved M, Urban M, Kello V, Diercksen GH (2001) Accuracy assessment of the ROHF-CCSD(T) calculations of static dipole polarizabilities of diatomic radicals: \({\text{O}}_2\), CN, and NO. J Mol Struct (THEOCHEM) 547:219–232
Miller WH, Handy NC, Adams JE (1980) Reaction path hamiltonian for polyatomic molecules. J Chem Phys 72:99–112
Minaev BF, Murugan NA, Agren H (2013) Dioxygen spectra and bioactivation. Int J Quantum Chem 113:1847–1867
Monge-Palacios M, Sarathy SM (2018) Ab initio and transition state theory study of the OH + \({\text{HO}}_2 \rightarrow {\text{H}}_2\)O + \({\text{O}}_2\)(\(^3\Sigma _g^-\))/\({\text{O}}_2\)(\(^1\Delta _g\)) reactions: yield and role of \({\text{O}}_2\)(\(^1\Delta _g\)) in \({\text{H}}_2{\text{O}}_2\) decomposition and in combustion of \({\text{H}}_2\). Phys Chem Chem Phys 20:4478–4489
Njegic B, Gordon MS (2006) Exploring the effect of anharmonicity of molecular vibrations on thermodynamic properties. J Chem Phys 125:224102
Ombrello T, Popov N (2015) Mechanisms of ethylene flame propagation enhancement by \({\text{O}}_2(a^1\Delta _g)\). Aerospace Lab, hal–01270947. https://doi.org/10.12762/2015.AL10-07
Pelevkin AV, Loukhovitski BI, Sharipov AS (2017) Reaction of H\(_2\) with O\(_2\) in excited electronic states: reaction pathways and rate constants. J Phys Chem A 121:9599–9611. https://doi.org/10.1021/acs.jpca.7b09964
Pelevkin AV, Sharipov AS (2018) Reactions of electronically excited molecular nitrogen with H\(_2\) and \({\text{H}}_2\)O molecules: theoretical study. J Phys D Appl Phys 51:184003
Pershin AA, Torbin AP, Zagidullin MV, Mebel AM, Mikheyev PA, Azyazov VN (2018) Rate constants for collision-induced emission of \({\text{O}}_2(a^1\Delta _g)\) with He, Ne, Ar, Kr, \({\text{N}}_2\), C\({\text{O}}_2\) and SF\(_6\) as collisional partners. Phys Chem Chem Phys 20:29677–29683
Petersson GA (2002) Quantum-mechanical prediction of thermochemical data. In: Cioslowski J (ed) Complete basis set models for chemical reactivity: from the helium atom to enzyme kinetics. Kluwer Academic Publishers, New York, pp 99–130
Polyansky OL, Ovsyannikov RI, Kyuberis AA, Lodi L, Tennyson J, Zobov NF (2013) Calculation of rotation-vibration energy levels of the water molecule with near-experimental accuracy based on an ab initio potential energy surface. J Phys Chem A 117:9633–9643
Popov NA (2016) Kinetics of plasma-assisted combustion: effect of non-equilibrium excitation on the ignition and oxidation of combustible mixtures. Plasma Sources Sci Technol 25:043002
Roos BO (1987) The complete active space self-consistent field method and its applications in electronic structure calculations. In: Lawley KP (ed) Advances in chemical physics: ab initio methods in quantum chemistry, part 2, vol 69. Wiley, Hoboken. https://doi.org/10.1002/9780470142943.ch7
Ruscic B, Bross DH (2016) Active thermochemical tables (ATcT) values based on ver. 1.122 of the thermochemical network. https://atct.anl.gov/. Accessed Dec 2018
Ryu SO, Shin KS, Hwang SM (2017) Determination of the rate coefficients of the \({\text{CH}}_4 + {\text{O}}_2 \rightarrow {\text{HO}}_2 + {\text{CH}}_3\) and \(\text{ HCO } + {\text{O}}_2 \rightarrow {\text{HO}}_2\) + CO reactions at high temperatures. Bull Korean Chem Soc 38:228–236
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347–1363. https://doi.org/10.1002/jcc.540141112
Schmidt MW, Gordon MS (1998) The construction and interpretation of MCSCF wavefunctions. Annu Rev Phys Chem 49:233–266
Schmidt R (1992) Collisional deactivation of \({\text{O}}_2(^1\Sigma _g^+)\) by small polyatomic molecules. Ber Bunsenges Phys Chem 96:794–799
Schweitzer C, Schmidt R (2003) Physical mechanisms of generation and deactivation of singlet oxygen. Chem Rev 103:1685–1757
Sharipov A, Starik A (2011) Theoretical study of the reaction of carbon monoxide with oxygen molecules in the ground triplet and singlet delta states. J Phys Chem A 115(10):1795–1803. https://doi.org/10.1021/jp110345s
Sharipov AS, Loukhovitski BI, Pelevkin AV, Kobtsev VD, Kozlov DN (2019) Polarizability of electronically excited molecular oxygen: theory and experiment. J Phys B At Mol Opt Phys 52:045,101
Sharipov AS, Loukhovitski BI, Starik AM (2017) Influence of vibrations of polyatomic molecules on dipole moment and static dipole polarizability: theoretical study. J Phys B At Mol Opt Phys 50:165101
Sharipov AS, Pelevkin AV (2019) On the reactivity of singlet delta oxygen with respect to the simplest hydrocarbons. Goren Vzryv (Mosk) Combust Explos 12:4–11 (in Russian, English abstract)
Sharipov AS, Starik AM (2012) Kinetic mechanism of CO–H\(_2\) system oxidation promoted by excited singlet oxygen molecules. Combust Flame 159:16–29. https://doi.org/10.1016/j.combustflame.2011.06.015
Sharipov AS, Starik AM (2012) Theoretical study of the reaction of ethane with oxygen molecules in the ground triplet and singlet delta states. J Phys Chem A 116:8444–8454. https://doi.org/10.1021/jp304906u
Sharipov AS, Starik AM (2013) Analysis of the reaction and quenching channels in a H + \({ \rm O}_2(a^1\Delta _g)\) system. Phys Scr 88:058305. https://doi.org/10.1088/0031-8949/88/05/058305
Sharipov AS, Starik AM (2015) Theoretical study of the reactions of ethanol with aluminum and aluminum oxide. J Phys Chem A 119:3897–3904. https://doi.org/10.1021/acs.jpca.5b01718
Silva-Junior MR, Schreiber M, Sauer SPA, Thiel W (2010) Benchmarks of electronically excited states: basis set effects on CASPT2 results. J Chem Phys 133:174,318
Simmie JM (2003) Detailed chemical kinetic models for the combustion of hydrocarbon fuels. Prog Energy Combust Sci 29(6):599–634
Slanger TG, Cosby PC (1988) \({\text{O}}_2\) spectroscopy below 5.1 eV. J Phys Chem 92(2):267–282
Smirnov VV, Stelmakh OM, Fabelinsky VI, Kozlov DN, Starik AM, Titova NS (2008) On the influence of electronically excited oxygen molecules on combustion of hydrogen–oxygen mixture. J Phys D Appl Phys 41:192001. https://doi.org/10.1088/0022-3727/41/19/192001
Srinivasan NK, Michael JV, Harding LB, Klippenstein SJ (2007) Experimental and theoretical rate constants for \({\text{CH}}_{4}+ {\text{O}}_{2} \rightarrow {\text{CH}}_{3} + {\text{HO}}_{2}\). Combust Flame 149:104–111. https://doi.org/10.1016/j.combustflame.2006.12.010
Starik A, Sharipov A (2011) Theoretical analysis of reaction kinetics with singlet oxygen molecules. Phys Chem Chem Phys 13:16424–16436. https://doi.org/10.1039/c1cp21269a
Starik AM, Bezgin LV, Kopchenov VI, Loukhovitski BI, Sharipov AS, Titova NS (2013) Numerical study of the enhancement of combustion performance in a scramjet combustor due to injection of electric-discharge-activated oxygen molecules. Plasma Sources Sci Technol 22(6):065007. https://doi.org/10.1088/0963-0252/22/6/065007
Starik AM, Kozlov VE, Titova NS (2010) On the influence of singlet oxygen molecules on the speed of flame propagation in methane–air mixture. Combust Flame 157(2):313–327. https://doi.org/10.1016/j.combustflame.2009.11.008
Starik AM, Kozlov VE, Titova NS (2014) Modeling study of the possibility of HCCI combustion improvement via photochemical activation of oxygen molecules. Energy Fuels 28:2170–2178
Starik AM, Loukhovitski BI, Sharipov AS, Titova NS (2015) Physics and chemistry of the influence of excited molecules on combustion enhancement. Philos Trans R Soc A 373:20140,341
Starik AM, Pelevkin AV, Titova NS (2017) Modeling study of the acceleration of ignition in ethane–air and natural gas–air mixtures via photochemical excitation of oxygen molecules. Combust Flame 176:81–93
Starik AM, Titova NS (2003) Possibility of intensifying chain reactions in combustible mixtures by laser radiation exciting electronic states of \({\text{O}}_2\) molecules. Dokl Phys 48:398–404
Starik AM, Titova NS (2006) Intensification of the oxidation of rich methane/air mixtures by \({\text{O}}_2\) molecules excited to the \(a^1\Delta _g\) state. Kinet Catal 47(4):487–496
Starikovskaia SM (2014) Plasma-assisted ignition and combustion: nanosecond discharges and development of kinetic mechanisms. J Phys D Appl Phys 47:353001
Starikovskiy A, Aleksandrov N (2013) Plasma-assisted ignition and combustion. Prog Energy Combust Sci 39:61–110. https://doi.org/10.1016/j.pecs.2012.05.003
Strelkova MI, Kirillov IA, Potapkin BV, Safonov AA, Sukhanov LP, Umanskiy SY, Deminsky MA, Dean AJ, Varatharajan B, Tentner AM (2008) Detailed and reduced mechanisms of jet a combustion at high temperatures. Combust Sci Technol 180:1788–1802
Sumathi R, Green WH Jr (2002) A priori rate constants for kinetic modeling. Theor Chem Acc 108:187–213
Szabo P, Gustafsson M (2017) A surface-hopping method for semiclassical calculations of cross sections for radiative association with electronic transitions. J Chem Phys 147:094,308
Takayanagi T (2002) Quantum scattering calculations of the O(\(^1D\)) + \({\text{N}}_2\)(X\(^1\Sigma _g^+\)) \(\rightarrow\) O(\(^3P\)) + \({\text{N}}_2\)(X\(^1\Sigma _g^+\)) spin-forbidden electronic quenching collision. J Phys Chem A 106:4914–4921
Topaler MS, Allison TC, Schwenke DW, Truhlar DG (1998) Test of trajectory surface hopping against accurate quantum dynamics for an electronically nonadiabatic chemical reaction. J Phys Chem A 102:1666–1673
Troe J (1977) Theory of thermal unimolecular reactions at low pressures. II. Strong collision rate constants. Applications. J Chem Phys 66:4758–4774
Truhlar DG (1991) A simple approximation for the vibrational partition function of a hindered internal rotation. J Comput Chem 12:266–270
Tsang W, Hampson RF (1986) Chemical kinetic data base for combustion chemistry. J Phys Chem Ref Data 15(3):1087–1280
Tully JC (2012) Perspective: nonadiabatic dynamics theory. J Chem Phys 137:22A301
Vagin NP, Kochetov IV, Napartovich AP, Yuryshev NN (2016) Acceleration of methane oxygen mixture ignition by adding singlet oxygen produced in a chemical generator. Bull Lebedev Phys Inst 43:211–216
Vagin NP, Kochetov IV, Napartovich AP, Yuryshev NN (2016) Influence of chemically produced singlet delta oxygen molecules on thermal ignition of \({\text{O}}_2\)–\({\text{H}}_2\) mixtures. J Phys D Appl Phys 49:055505
Wang X, Ding S, Wang P, Xie J, Zhong W (2005) Algebraic approach to the potential energy surface and vibration energy of the transition molecule-HO\(_2\). Chin J Phys 43:1051–1057
Weltmann KD, Kolb JF, Holub M, Uhrlandt D, Šimek M, Ostrikov K, Hamaguchi S, Cvelbar U, Černák M, Locke B, Fridman A, Favia P, Becker K (2019) The future for plasma science and technology. Plasma Process Polym 16:e1800118. https://doi.org/10.1002/ppap.201800118
West AC, Kretchmer JS, Sellner B, Park K, Hase WL, Lischka H, Windus TL (2009) O(\(^3P\)) + \(\text{ C }_2{\text{H}}_4\) potential energy surface: study at the multireference level. J Phys Chem A 113:12663–12674
Woon DE, Dunning TH (1994) Gaussian basis sets for use in correlated molecular calculations. IV. Calculation of static electrical response properties. J Chem Phys 100:2975–2988
Yamaguchi Y, Teng Y, Shimomura S, Tabata K, Suzuki E (1999) Ab initio study for selective oxidation of methane with NO\(_x\) (\(x=1,2\)). J Phys Chem A 103:8272–8278
Zagidullin MV, Khvatov NA, Tolstov GI, Medvedkov IA, Mebel AM, Heaven MC, Azyazov VN (2018) \({\text{O}}_2(^1\Sigma _g^+)\) removal by \({\text{H}}_2\), CO, \({\text{N}}_2\)O, \({\text{CH}}_4\), and \(\text{ C }_2{\text{H}}_4\) in the 300–800 K temperature range. J Phys Chem A 122:5283–5288
Zavitsas AA (1987) Quantitative relationship between bond dissociation energles, infrared stretching frequencies, and force constants in polyatomic molecules. J Phys Chem 91:5573–5577
Zhang F, Huang C, Xie B, Wu X (2019) Revisiting the chemical kinetics of \({\text{CH}}_3 + {\text{O}}_2\) and its impact on methane ignition. Combust Flame 200:125–134
Zhang J, Hu T, Lv H, Dong C (2016) H-abstraction mechanisms in oxidation reaction of methane and hydrogen: a CASPT2 study. Int J Hydrogen Energy 41:12722–12729
Zhu R, Lin MC (2001) The \({\text{CH}}_3 + {\text{HO}}_2\) reaction: first-principles prediction of its rate constant and product branching probabilities. J Phys Chem A 105:6243–6248. https://doi.org/10.1021/jp010698i
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (Projects Nos. 17-01-00810 and 17-08-01423).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest. Also we declare that both the co-authors are aware of and approve of the submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pelevkin, A.V., Sharipov, A.S. Interaction of CH4 with Electronically Excited O2: Ab Initio Potential Energy Surfaces and Reaction Kinetics. Plasma Chem Plasma Process 39, 1533–1558 (2019). https://doi.org/10.1007/s11090-019-10008-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-10008-7