Abstract
The objective of this study was to determine the influence of low-temperature plasma (LTP) on seed surface modification, water uptake by seeds, seed germination and vigor of seedlings, as well as changes in the content of endogenous hormones in pea, (Pisum sativum L. var. Prophet). The study’s authors used diffuse coplanar surface barrier discharge as the source of LTP in various duration times of treatment (from 60 to 600 s). The SEM analysis showed that LTP induced significant changes on the seeds’ surface, which was related to water permeability into the seeds. LTP increased the germination percentage of pea seeds as well as the growth parameters (root and shoot length, dry weight), and the vigor of seedlings and the effects of LTP also depended on exposure time. The LTP-pretreatment produced changes in endogenous hormones (auxins and cytokinins and their catabolites and conjugates), which correlated with increased growth of the pea seedlings. The results suggested an interaction among the modification of seed structure demonstrated by LTP in the induction of faster germination and hormonal activities related to plant signaling and development during the early growth of pea seedlings.
Similar content being viewed by others
Introduction
The importance of physical methods in biological and agricultural research have been presented in [1]. Physical factors such as ionizing radiation, high electromagnetic field, microwave irradiation and cold atmospheric pressure plasma play an important role not only in medicine for bacterial decontamination [2], but are also useful for industrial purposes, e.g. for increasing surface hydrophilicity and material surface modification [3]. A promising physical method that offers a broad range of interesting industrial applications is low-temperature plasma (LTP) generated by diffuse coplanar surface barrier discharge (DCSBD) [4]. The considerable effect of LTP for the disinfection and sterilization of a wide range of materials was observed [5, 6], and the effective consequence of plasma treatment is also the inactivation and destruction of microflora occurrence on the surface of plant seeds and food [7–9]. Plasma is partially ionized gases also known as a highly-energized fourth state of matter that contains ions, electrons, and reactive neutral particles (radicals, and excited atoms and molecules), and sometimes with sufficient energy to break covalent bonds and/or initiate various chemical reactions [10]. The highly reactive environment of LTP according to Larrousi [11] may lead to seed surface modifications and potential disruptions [12].
One of the potential areas of low-temperature plasma research can be plant cultivation, especially pre-sowing treatment by LTP technique [13]. Some studies showed that LTP can increase seed activity including earlier germination, higher germination rate, faster growth [14, 15], enzyme activity [16, 17], and the yield of plants [18]. For instance, air plasma treatment also changes the wetting properties of seeds due to oxidation of their surface that leads to faster germination and greater yield of wheat and oats [14] and lentils, beans and wheat [19]. For seed germination and seed viability, studies of plants were carried out using different plasma sources [20, 21], that create different concentrations of plasma reactive species, charged particles and photons.
It is well known that in the process of seed germination and ontogenetic development of plants, a significant role is played by endogenous plant hormones [22]. Cytokinins and auxins affect cell division, cell elongation, cell differentiation factors and cell type specific gene [23], thus both cytokinins and auxins are important in seed germination and the early growth of plants [24]. Cytokinins that control plant growth and development were first identified as a factor that induces cell division in the presence of auxins [25, 26]. The influence of low-temperature plasma on the biosynthesis and content of endogenous phytohormones in plants has not yet been studied. Therefore, it is a question whether the application of plasma on seeds also affects the hormonal status of pea plants. In this study the seeds of Pisum sativum, commonly known as pea, were used. This leguminous food is a rich source of carbohydrate, protein, vitamins and minerals [27]. In this paper, the low-temperature plasma application for seed surface modification and changes in the dynamics of water uptake into the pea seeds is reported. The authors also investigate the optimization of the operating conditions in low-temperature plasma to increase the percentage germination and total vigor of seedlings. In this work, the current authors also suggest an interaction among the LTP and some endogenous hormones during the early growth of pea seedlings.
Materials and Methods
Plant Material
In the experiments the current authors used the dried seeds of pea (Pisum sativum L.) var. Prophet (obtained from the Central Controlling and Testing Institute of Agriculture in Bratislava, Slovakia) which were stored at 10 °C in the dark.
Characteristics of Plasma Source
The plasma treatment of pea seeds was performed by DCSBD, a planar source of low-temperature plasma working at atmospheric-pressure in ambient air [3]. The DCSBD electrode geometry consists of numerous parallel strip-like silver electrodes embedded 0.5 mm below the surface of 96 % Al2O3 ceramics. The DCSBD generates on an alumina plate a thin uniform large-area layer of macroscopically homogeneous non-equilibrium plasma at a plasma power density of above 100 W cm−2. The discharge was powered by 14 kHz sinusoidal voltage with an amplitude of approximately 10 kV, supplied by HV plasma power supply. The electrical parameters of discharge were monitored by the current monitor Model 4100 (Pearson Electronics) and two high voltage probes Tektronix P6015A (1:1000). The signals from all three electrical probes were recorded by the digitizing oscilloscope Tektronix TDS 2014B. The input energy of 370 W was calculated from measured current–voltage waveforms, and the average plasma power density was 2.3 W cm−2. The total power consumed by plasma was used for plasma processing, which allowed for short treatment times and the possible incorporation of the DCSBD-plasma source directly in the continuously working production lines. The basic properties of such DCSBD discharge in the air were previously described in detail by Černák et al. [4].
Plasma Treatment of Seeds
The plasma treatment of seeds was performed at input power 370 W (calculated from voltage and current waveforms measurement), when the entire DCSBD electrode is covered with a plasma layer and all seeds are treated homogeneously. As depicted (Fig. 1) the pea seeds were placed in the plasma layer on ceramic, and the movement of seeds on its surface was carried out mechanically to ensure uniform treatment. For experiments, the pea seeds were treated with plasma exposure times ranging from 60 to 600 s. The seeds were taken from the plasma field after treatment, and exposed to the atmosphere for 24 h before the biological experiments started.
Growth Conditions
Control-untreated and treated seeds 24 h after plasma treatment were sown in experimental pots containing perlite substrate, with 100 seeds per variant in four repetitions. The moisture level of substrate was adjusted at 2 days intervals with Hoagland nutrient solution to avoid changes due to evaporation. The plants were cultivated for 7, 14 and 21 days in controlled growth conditions: 25 °C in the light and 18 °C in the dark, 12 h light/12 h dark photoperiod with a photon flux density of 120 µmol m−2 s−1 and 70 % air humidity. Germination-percentage, growth parameters and vigor seedlings were determined 7 days after sowing. The twenty plants from each treatment in four repetitions including control variant were harvested, and a perlite substrate was washed from the roots, and the length of roots and shoots of seedlings was measured. Plant material for dry weight was dried at 80 °C for 120 h. The percentage of germination was calculated using the equation:
and seed vigor I and II were calculated in modification according to [28], using the following equation:
Sample Preparation for Scanning Electron Microscopy (SEM)
Pea seeds were exposed to LTP for 0 (control sample), 120 and 600 s. Then the seeds were split into two halves, and the part with the embryo was fixed into an aluminum stand with electrically-conducting tape. The samples were dried in a vacuum kiln (JEOL EM/DSC 10, Japan) and carbon-resurfaced in a vacuum steamer. The samples were observed with scanning electron microscope SEM (JEOL JXA-840 A, Japan) with 15 kV accelerating voltage; we used 2000× magnification to observe the testal area near plumule and radicle apices. To observe the specific structures on seed surfaces, we used 70× and 1000× magnification.
Plasma Effect on Water Uptake
Seeds were exposed to LTP for 0 (control sample), 60, 120 and 180 s, and 100 seeds for one variant in three repetitions were used. The seeds were weighed on an electronic balance (Sartorius BL-210S, Germany) immediately after plasma treatment (time t0) and then in 2 h intervals (t0 + 2; +4; +6 and +8 h) during the imbibition processes (seeds were hydrated with distilled water) and incubated at 25 °C. Immediately after the initial wetting and at 2 h intervals, the seeds were blotted dry and weighed to the nearest 0.1 mg. The amount of water uptake was determined as the actual increase in seed weight. Water content per seed was measured and expressed according to the following equation:
where FW is fresh weight and DW is dry weight.
Endogenous Auxins and Cytokinins Determination
Pea seeds were exposed to LTP for 0 (control sample), 120 and 600 s, and cultivated for 1, 2 and 3 weeks in controlled conditions. The pea leaves of whole plants were collected, cut into small segments, homogenized, and 50 mg of leaf tissues were used in four repetitions for each time point. The samples were then frozen in liquid nitrogen and stored at −70 °C. Phytohormones extraction, purification and quantification were performed according to [29–33] with modifications. For each extract, 0.5 pmol and 1 pmol of stable isotope labeled cytokinin bases/ribosides/N-glucosides and O-glucosides/nucleotides (Olchemim), and 5 pmol of [13C6]IAA/[13C6]IAAsp/[13C6]IAGlu/[13C6]oxIAA (Cambridge Isotope Laboratories) were added as internal standards to check recovery during purification and to validate the quantification, respectively. Auxins and cytokinins were determined using the liquid chromatography-tandem mass spectrometry system, consisting of an Acquity UPLCTM system (Waters, USA) and XevoTM TQ (Waters Technologies, UK) tandem quadrupole mass spectrometer. The concentration of various phytohormone metabolites was calculated based on the standard isotope dilution method [34]. Data were processed with Masslynx™ software (version 4.1, Waters, USA) using QuanLynx™ program.
Statistical Analysis
The results were processed in Microsoft Excel and the Statgraphics Plus 5.1 and Statgraphics XV. Centurion statistical programs. All data obtained were subjected to a one-way analysis of variance (ANOVA), and the mean differences were compared by lowest standard deviations (LSD) test. Comparisons with P ≤ 0.05 were considered significantly different. In the figures, the spread of values is shown as error bars representing standard errors of the means.
Results
SEM Analysis
Scanning electron microscopy (SEM) showed that LTP significantly modifies the seed surface structures of pea, and morphological differences depending on observed testal area were also observed (Figs. 2, 3). Disruption, abrasion or even the loosening of original structures in testal areas both near the plumule apex (Fig. 2) and radicle apex (Fig. 3) was observed. These changes occurred as soon as after 120 s of LTP treatment, but the most striking changes were visible after 600 s exposure to LTP in both observed areas of testa (Figs. 2c, 3c) where splits and rifts could be seen as well as (Fig. 4b arrowed). It was also observed that LTP is only effective a certain distance from the discharge tube surface and modifies mainly protruding parts (Fig. 4a—marked by “1”) of seed, while depressed parts are less modified (Fig. 4a—“2”). Figure 4b shows the gradual transition between more (“1”) and less (“2”) modified testal areas; a rift in the testal surface is arrowed.
SEM photographs of pea testal surface after 600 s LTP treatment. In both a, b photographs No. 1 marks protruding areas modified by LTP and No. 2 marks surface depressed parts, less modified by LTP. In b photograph is see of gradual transition between more and less modified testal areas. The arrows in the b indicate craks in the seed coat
Influence of LTP on Water Uptake
The findings suggest an acceleration in the water uptake of pea seeds treated by low-temperature plasma. It was shown that higher and more intensive water uptake positively correlates with the increasing exposure time of LTP. In the case of 180 s LTP application dose, the most striking difference was visible after the first 2 h, when the seeds absorbed 23.3 % more water compared to the control (Fig. 5). In contrast, longer imbibition time causes a decrease in the dynamics of water uptake in plasma treated seeds. These findings suggest that plasma treated seeds reached water-saturation significantly faster compared to untreated seeds. After 8 h the seeds were mostly well-watered in all variants, and there were no significant differences among the variants.
Influence of LTP on Growth Parameters
The results for treatment times ranging between 60 and 180 s are depicted (Table 1). After 7 days treatment in a LTP field a different, but across all experiments, significant stimulating effect on the germination, shoot and root length, dry weight as well as vigor of pea seedlings (Table 1) was found. The total percentage of seed germination significantly increased for LTP treatment time of up to 120 s (95 vs. 77.5 %), shoot and root length increased from (4.7 and 13.5 vs. 4.1 and 12.0 cm), the seedling dry weight of (57.7 vs. 53.9 mg) and the vigor index I and II from (17.3 and 54.8 vs. 12.5 and 41.8) compared to the control samples.
Influence of LTP on Endogenous Phytohormones Content
IAA Content, its Catabolites and Conjugates
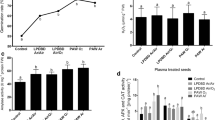
In the experiments, the content of endogenous IAA, its catabolite oxidized form (oxIAA) and also its conjugates (IAAsp and IAGlu) were determined. It was revealed that in 7 days old pea seedlings the content of IAA increased insignificantly (by 13.7 %) after 120 s LTP treatment in comparison to untreated control (Fig. 6). In the case of 14 days old seedlings, no statistically significant changes dependent on LTP application dose compared to the control (Table 2) were observed either. In 7 days old seedlings, the level of oxIAA decreased significantly depending on increased LTP exposure time, and after the 600 s treatment oxIAA content decreased by 43.8 % (Fig. 6). In contrast, in 14 days old seedlings the level of oxIAA increased significantly with higher LTP exposure time, reaching a value after the 600 s treatment of 11.45 pmol g−1 f.w., an increase of 64.3 % in comparison with the control. Even in the 21 days old seedlings the oxIAA concentration shows a similar tendency as in the previous case, in comparison to the control and for the 600 s treated seedlings oxIAA content increased by 73.3 % (Table 2). In 7 days old seedlings, the levels of IAA conjugates changed considerably, and the concentration of IAAsp decreased with higher LTP exposition time compared to the control. In the 120 and 600 s application doses, a decrease by 29 and 86.3 % was revealed (Fig. 6). In the 14 days old seedlings, no significant changes of this compound content were observed, despite the fact that after 120 s plasma treatment it was 5 % higher in comparison to the control. Similarly, there were no significant changes in the 21 days old seedlings, although in 600 s plasma treated plants the content of IAAsp was 16.6 % higher than the control sample (Table 2). Moreover, it was observed that in the 7 days old seedlings the IAGlu concentration was the highest after 120 s treatment by low-temperature plasma, and this increase was significantly higher by 54.7 % (Fig. 6) in comparison to the control plants. In the 14 and 21 days old seedlings, no significant changes in IAGlu content was recorded.
Effect of a LTP exposure at different time interval (120 and 600 s) on concentration of indolylacetic acid (IAA), oxidized form of IAA (oxIAA), indole-acetyl aspartate (IAAsp) and indole-acetyl glutamate (IAAGlu) in 7 days old pea seedlings. Values in the figure are mean ± SE (n = 4). The values marked by different letter are statistically significant at P ≤ 0.05 according to LSD ANOVA
Cytokinins Content
Given the high number of analyzed cytokinins, as well as the fact that their concentrations were determined in the 7, 14, and 21 days old seedlings, mainly the statistically changed levels of cytokinins are shown in the charts. In the case of 7 days old seedlings, a non-significant increase of trans-zeatin (tZ) depending on higher LTP exposure time was revealed, despite the fact that after 120 and 600 s treatment its level was 40 and 52.6 % higher in comparison to the control samples (Fig. 7). Similarly, the level of trans-zeatin-O-glucoside (tZOG) did not statistically change, although after 120 s treatment it was 43.6 % higher than the control. The level of trans-zeatin riboside (tZR) significantly increased depending on the higher plasma application dose, and after 120 and 600 s of LTP exposure it was significantly higher by 158.2 and 264.1 % than the control (Fig. 8). It was also revealed that increasing the LTP application dose causes a higher concentration of trans-zeatin riboside-O-glucoside (tZROG), as after the 120 s treatment the level of this compound was significantly higher by 119.2 % and after the 600 s exposition it was significantly higher by 71.85 % in comparison to the control samples (Table 3). The experiments revealed no changes in the levels of cytokinin cis-forms after low-temperature plasma treatment in 7 days old plants. However, LTP significantly fortifies the dihydrozeatin riboside (DHZR) level. After the 120 s LTP treatment, the level of DHZR increased by 98.6 % in comparison to the control, and after the 600 s exposure time it reached 154 % higher concentration than in the control (Fig. 7). The concentration of dihydrozeatin riboside-O-glucoside (DHZROG) also increased significantly compared to the untreated samples, namely by 107.3 % after 120 s LTP treatment and by 179 % after 600 s LTP exposure time (Fig. 8). Moreover, the level of isopentenyladenine riboside (iPR) was higher after the plasma treatment, being 46.1 and 44.7 % higher than the control seedlings after the 120 and 600 s. treatment, respectively. The changes in isopentenyladenosine riboside-5′-monophosphate (iPR-5′-MP) concentrations was revealed, when after 120 s plasma treatment such increased by 13.7 % compared to the control. In contrast, after 600 s of LTP exposition they were 14.7 % lower than the control (Fig. 8). The experiments showed a different pattern of cytokinin concentrations in 14 days old pea seedlings compared to the 7 days old ones. It is understood that this phenomenon is due to many metabolic processes, substitutions in functional groups, etc. According to the amount of output data, only statistically significantly changed or plant-development important compounds are shown. The concentration of trans-zeatin (tZ) was significantly higher only after the 600 s LTP exposition, but there was no significant difference between the control samples and the 120 s treated samples (Fig. 8). The trans-zeatin-O-glucoside (tZOG) level was the lowest after the 120 s LTP exposure time against the 600 s variant, where this compound content peaked (Fig. 8). The level of endogenous tZR significantly decreased with increasing LTP application dose, and its highest concentration in the control samples was present (Fig. 8). The same pattern was observed in the case of tZROG (Table 3). Cis-zeatin riboside-5′-monophosphate (cZR-5′-MP) reflected higher concentration after the 120 s plasma treatment, but after the 600 s exposition it decreased significantly in comparison to the 120 s variant. A very similar tendency was revealed in the dihydrozeatin riboside-O-glucoside (DHZROG) concentrations (Fig. 8). The level of dihydrozeatin (DHZ) was significantly the highest after the 600 s treatment in comparison to the control and the 120 s variant (Fig. 9). Plasma treatment did not cause any changes in the dihydrozeatin riboside (DHZR) concentration. The isopentenyladenine riboside (iPR) concentration peaked after the LTP highest application dose in comparison to the control (Table 3), but the level of isopentenyladenosine-5′-monophosphate (iPR-5′-MP) was the highest after 120 s of low-temperature plasma exposition (Fig. 8). In 21 days old seedlings, changes in cytokinins concentration were also reflected compared to the previous variant. Trans-zeatin (tZ) peaked in LTP-untreated samples, with the rising LTP application dose causing its decrease. Trans-zeatin-O-glucoside (tZOG) content remained unchanged regardless of plasma application dose (Fig. 9). The lowest content of trans-zeatin riboside-O-glucoside (tZROG) was observed in the control samples, but in both application doses of LTP it was significantly higher (Table 3). In the case of trans-zeatin riboside (tZR), there were no changes between the control and the 120 s treated variant, but its level significantly decreased after the 600 s LTP application dose. Cis-zeatin riboside-O-glucoside (cZROG) content peaked after the 120 s LTP treatment, and it significantly differed from the other two variants (Table 3). The dihydrozeatin (DHZ) concentration simultaneously decreased according to the growing LTP application dose (Fig. 9). The level of dihydrozeatin riboside (DHZR) peaked significantly in the 120 s treated variant, but then decreased, and the dihydrozeatin riboside-O-glucoside (DHZROG) content in both plasma-treated samples significantly increased, in contrast to the control seedlings (Fig. 9). It was also observed that the level of isopentenyladenine (iPR) was the lowest after the 600 s plasma treatment, but peaked after the 120 s application dose (Table 3). The highest level of isopentenyladenosine-5′-monophosphate (iPR-5′-MP) was present in the 600 s plasma treated variant, and this value was significantly different from the control and the 120 s LTP-treated variant (Fig. 9).
Effect of a LTP exposure at different time interval (120 and 600 s) on concentration of trans-zeatin (tZ), trans-zeatin-O-glucoside (tZOG), trans-zeatin riboside (tZR), dihydrozeatin riboside (DHZR), dihydrozeatin riboside-O-glucoside (DHZROG) and isopentenyladenosine riboside-5′-monophosphate (iPR-5′-MP) in 7 days old pea seedlings. Values in the figure are mean ± SE (n = 4). The values marked by different letter are statistically significant at P ≤ 0.05 according to LSD ANOVA
Effect of a LTP exposure at different time interval (120 and 600 s) on concentration of trans-zeatin (tZ), trans-zeatin-O-glucoside (tZOG), trans-zeatin riboside (tZR), cis-zeatin riboside-5′-monophosphate (cZR-5′-MP), dihydrozeatin (DHZ), dihydrozeatin riboside (DHZR), dihydrozeatin riboside-O-glucoside (DHZROG) and isopentenyladenosine riboside-5′-monophosphate (iPR-5′-MP) in 14 days old pea seedlings. Values in the figure are mean ± SE (n = 4). The values marked by different letter are statistically significant at P ≤ 0.05 according to LSD ANOVA
Effect of a LTP exposure at different time interval (120 and 600 s) on concentration of trans-zeatin (tZ), trans-zeatin-O-glucoside (tZOG), trans-zeatin riboside (tZR), dihydrozeatin (DHZ), dihydrozeatin riboside (DHZR), dihydrozeatin riboside-O-glucoside (DHZROG) and isopentenyladenosine riboside-5′-monophosphate (iPR-5′-MP) in 21 days old pea seedlings. Values in the figure are mean ± SE (n = 4). The values marked by different letter are statistically significant at P ≤ 0.05 according to LSD ANOVA
Discussion
This research on the influence of low-temperature plasma on pea seeds led to both similar and different conclusions in comparison with other authors’ results concerning the issue of LTP influence on plant material. As well as the SEM analysis of the seed surface and membrane permeability and water uptake by seeds, the effect of different LTP treatment times on the germination, growth parameters and vigor of pea seedlings were also investigated. Significant modifications of pea seed surface were observed under LTP influence, an effect that is enhanced by the increasing application exposure time of plasma. As the results showed, the seed coat can be eroded by plasma and therefore it was supposed that the erosion of the hydrophobic wax layer on the pea seed coat by LTP caused the change in water permeability that corresponded with the results of Tong et al. [21] in Andrographis paniculata treated by atmospheric pressure air plasma. Also the results of Dhayal et al. [12], who modified safflower (Carthamus tinctorius L.) seed surfaces by treating with a low pressure (l6 Pa) radiofrequency (20 W) argon gas discharge, achieved a significant physical structure modification of the seed coat and hilum of safflower seeds. Based on the findings, it is also supposed that the significant modifications and disruptions of pea seed surfaces are directly related not only to the loss of seed weight (data not shown) but also to faster imbibition processes. Furthermore, a loss of cell membrane integrity also causes, according to [9], an increase in leachate conductivity. After the treatment of pea seeds, significant changes of the surface structure and seed coat increased the uptake and permeability of water into the seed. Since the hydrophilic of seeds was improved by LTP treatment, the ability of seeds or plants respectively to absorb water and nutrition was relatively enhanced, leading to better growth in correspondence with the results of [15]. Bormashenko et al. [19] also report the possible wetting of the surfaces of a diversity of lentils, beans and wheat seeds with cold radiofrequency air plasma treatment. The change in the wetting properties of seeds is at least partially due to the oxidation of their surface under plasma treatment, and the change in the wettability of seeds gives rise to a change in water absorption (imbibition) of these seeds.
The results showed that due to the low-temperature plasma treatment times ranging from 120 to 180 s., the seed membrane permeability of pea was changed, resulting in the acceleration of seed germination and seedling vigor. The vigor index accumulates both features of germination, as well as root and shoot length and the dry weight of seedlings. The results indicate a stimulating influence of LTP on germination and growth parameters mainly after the 120 s treatment. These results correspond with the results of Muraji et al. [35] which reported that only relatively low exposure time of magnetic field stimulated the growth of corn roots, whereas higher doses above 300 s inhibited growth. Stimulatory or inhibitory LTP effect on the growth of different plant species depend not only on the exposure time of plasma, but also on the plasma source used. The results described in this paper correlate convincingly with [36] in case of magnetic field-treated seeds of cucumber. In this paper, the mechanism behind the observed increased germination is not completely understood, but we showed that the seeds’ physiological and biochemical activity increases due to LTP treatment for 2 min. After 2 min., the physiological effect slightly decreased again. This could be due to an overdose of reactive oxygen and nitrogen species produced by the LTP, as such have a significant role in the regulation of endogenous hormones not only during seed imbibition but also in the early development stage of pea plants [37]. The authors suspect that changes in the hormonal activity of seedlings could obscure potential differences in root and shoot length increase, as well as in the dry weight of seedlings. Simultaneously, the penetration of reactive compounds and charged particles, as well as UV irradiation and ozone from the LTP environment into the seed [11] suggest that also in the case of pea seeds, plants can modify some metabolic, biosynthetic and signaling pathways [38]. It is assumed that hormone level changes can be considered under the influence of low-temperature plasma on young pea seedlings. It is also supposed that increasing auxin levels in synergy with cytokinins can stimulate cell division and proliferation as well as cell elongation. The verity of the hypothesis is supported by the present authors’ results of seed vigor index, evaluated on the basis of root and shoot lengths after LTP treatment. The changing concentrations of conjugates and metabolites derived from auxins, and especially from cytokinins, can be explained by the fact that in 7 days old seedlings a higher biosynthesis of biologically active forms is present which are then changed to conjugates and metabolites in later developmental stages. These compounds may, if necessary, serve as a deposit and source of biologically active forms [39]. However, Fridman et al. [2] assume that it is possible to connect the LTP’s influence on plants through several grow factors in which phytohormones are included. The increased concentration of auxin and their catabolites and conjugates after LTP treatment may also act either by turning on the expression of certain genes or by being involved in the modification of key gene products [40]. The data presented by the present authors herein confirmed that the influence of LTP increases the biosynthesis of some auxins and cytokinins, as well as their catabolites and conjugates. It is therefore considered that they can potentiate the synergy and their physiological effect, which can lead to improvements in the seed germination, growth and yield of agriculturally important plants [41]. Nevertheless, the exact function of phytohormones in plants after LTP treatment remains not entirely clear, and further experiments of later developmental stages in pea plants are required.
Conclusions
Seed germination, seedling growth and the biochemical parameters based on endogenous hormones (auxins and cytokinins) in the seedlings of P. sativum changed after pretreatment with low-temperature plasma. Seed water uptake was significantly increased in treated seeds, which helped the faster hydratation and activation of germination enzymes and phytohormones, and triggered metabolic events that led to the enhanced vigor of treated seeds. It was also concluded that the modification of pea seed surface area results in the interaction of electrons and ions of plasma with the outer layer of biological tissue. The results of the present authors showed that LTP affects not only seed surface modifications, but is probably also involved in several biochemical pathways inside the seed, which are reflected in changes in the growth and development of pea plants. On the basis of those findings, plasma has shown potential promise as a tool for commercial use in agricultural praxis.
Abbreviations
- DCSBD:
-
Diffuse coplanar surface barrier discharge
- DW:
-
Dry weight
- FW:
-
Fresh weight
- LTP:
-
Low-temperature plasma
- SEM:
-
Scanning electron microscopy
- HV:
-
High voltage
References
Bari ML, Nazuka E, Sabina Y et al (2003) Chemical and irradiation treatments for killing Escherichia coli O157:H7 on alfalfa, radish, and mung bean seeds. J Food Prot 66:767–774
Fridman G, Friedman G, Gutsol A et al (2008) Applied plasma medicine. Plasma Process Polym 5:503–533
Šimor M, Ráheľ J, Vojtek P et al (2002) Atmospheric-pressure diffuse coplanar surface discharge for surface treatments. Appl Phys Lett 81:2716–2718
Černák M, Černáková Ľ, Hudec I et al (2009) Diffuse coplanar surface barrier discharge and its applications for in-line processing of low-added-value materials. Eur Phys J Appl Phys 47(22806):p1–p6
Machala Z, Chládeková L, Pelach M (2010) Plasma agents in bio-decontamination by dc discharges in atmospheric air. J Phys D Appl Phys 43(222001):7
Uhm HS, Choi EH, Cho GS et al (2013) Influence of reactive oxygen species on the sterilization of microbes. Curr Appl Phys 13:530–535
Basaran P, Basaran-Akgul N, Oksuz L (2008) Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol 25:626–632
Selcuk M, Oksuz L, Basaran P (2008) Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Biores Technol 99:5104–5109
Mitra A, Li YF, Klämpfl TG et al (2014) Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioprocess Technol 7:645–653
Moisan M, Barbeau J, Crevier MC et al (2002) Plasma sterilization: methods and mechanisms. Pure Appl Chem 74:349–358
Larrousi M (2005) Low temperature plasma-based sterilization: overview and state-of-the art. Plasma Process Polym 2:391–400
Dhayal M, Lee SY, Par SU (2006) Using low-pressure plasma for Carthanus tinctorium L. seed surface modification. Vacuum 80:499–506
Ito M, Ohta T (2012) Plasma agriculture. J Korean Phys Soc 60:937–943
Šerá B, Špatenka P, Šerý M et al (2010) Influence of plasma treatment on wheat and oat germination and early growth. IEEE Trans Plasma Sci 38:2963–2968
Jiang J, He X, Li L et al (2014) Effect of cold plasma treatment on seed germination and growth of wheat. Plasma Sci Technol 16:54–58
Henselová M, Slováková Ľ, Martinka M et al (2012) Growth, anatomy and enzyme activity changes in Zea mays L. roots induced by treatment of seeds with low-temperature plasma. Biologia 67:490–497
Surowsky B, Fischer A, Schlueter O et al (2013) Cold plasma effects on enzyme activity in a model food system. Innov Food Sci Emerg Technol 19:146–152
Yin MQ, Huang MG, Ma BZ et al (2005) Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield. Plasma Sci Technol 7:3143–3147
Bormashenko E, Grynyov R, Bormashenko Y et al. (2012) Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci Rep 4:741–748
Dubinov AE, Lazarenko EM, Selemir VD (2000) Effect of glow discharge air plasma on grain crops seed. IEEE Trans Plasma Sci 28:180–183
Tong J, He R, Zhang X et al (2014) Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of Andrographis paniculata. Plasma Sci Technol 16:260–266
Cakmak I, Marschner H, Bangert F (1989) Effect of zinc nutritional status on growth, protein metabolism and levels of indole-3-acetic acid and other phytohormones in bean (Phaseolus vulgaris L.). J Exp Bot 40:404–412
Johri MM, Mitra D (2001) Action of plant hormones. Curr Sci 80:199–205
Singh S, Sawhney VK (1992) Endogenous hormones in seeds, germination behaviour and early seedling characteristics in a normal and ogura cytoplasmic male sterile line of rapeseed (Brassica napus L.). J Exp Bot 473:1497–1505
Miller CO, Skoog F, von Saltza HM et al (1955) Kinetin: structure and synthesis of kinetin. J Am Chem Soc 77:2662–2663
Letham DS (1994) In: Mok DWS, Mok MC (eds) Cytokinins: chemistry, activity and function. CRC Press, Raton
Costa GE, Queiroz-Monici K, Reis S, Oliveira AC (2006) Chemical composition of dietary fiber and resistant starch contents of raw and cooked pea, common bean, chickpea, and lentil legumes. Food Chem 94:327–330
Abdul-Baki AA, Anderson JD (1973) Vigour determination in soybean seed by multiplication. Crop Sci 3:630–633
Dobrev PI, Kamínek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr 950:21–29
Dobrev PI, Havlíček L, Vágner M et al (2005) Purification and determination of plant hormones auxin and abscisic acid using solid phase extraction and two-dimensional high performance liquid chromatography. J. Chromatogr 1075:159–166
Novák O, Hauserová E, Amakorová P et al (2008) Cytokinin profiling in plant tissues using ultra-performance liquid chromatography-electrospray tandem mass spectrometry. Phytochemistry 69:2214–2224
Novák O, Hényková E, Sairanen I et al (2012) Tissue-specific profiling of the Arabidopsis thaliana auxin metabolome. Plant J 72:523–536
Svačinová J, Novák O, Plačková L et al (2012) A new approach for cytokinin isolation from Arabidopsis tissues using miniaturized purification: pipete tip solid-phase extraction. Plant Methods 8:8–17
Rittenberg D, Foster L (1940) A new procedure for quantitative analysis by isotope dilution, with application to the determination of amino acids and fatty acids. J Biol Chem 133:727–744
Muraji M, Asai T, Tatebe W (1998) Primary root growth rate of Zea mays seedlings grown in alternating magnetic field of different frequencies. Bioelectrochem Bioenerg 44:271–273
Bhardwaj J, Anand A, Nagarajan S (2012) Biochemical and biophysical changes associated with magnetopriming in germinating cucumber seeds. Plant Physiol Biochem 57:67–73
Kranner I, Roach T, Beckett RP et al (2010) Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J Plant Physiol 167:805–811
Vashisth A, Nagarajan S (2008) Exposure of seeds to static magnetic field enhances germination and early growth characteristics in chickpea (Cicer arietinum L.). Bioelectromagnetics 29:571–578
Taiz LE, Zeiger E (2010) Plant physiology. Sinauer Associates, Sunderland
Liu W, Hildebrand DF, Scoott Grayburm W et al (1991) Effects of exogenous auxins on expression of lipoxygenases in cultured soybean embryos. Plant Physiol 97:969–976
Marinković D, Borcean I (2009) Effect of cold electron plasma and extremly low frequency electron-magnetic field on wheat yield. Res J Agric Sci 41:96–101
Acknowledgments
The authors wish to thank the Central Controlling and Testing Institute of Agriculture in Bratislava, Slovakia, for the samples of seeds. The authors are also thankful to Ing. Mária Čaplovičová for the SEM imaging of seeds and Mr. Darren Chastney for critically reviewing the manuscript. This study was supported by the Slovak Grant Agency for Science VEGA No.1/0904/14; by the Slovak Research and Development Agency, Contract No. APVV-14-0264 and by Grant LO1204 from the National Program of Sustainability I, Czech republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stolárik, T., Henselová, M., Martinka, M. et al. Effect of Low-Temperature Plasma on the Structure of Seeds, Growth and Metabolism of Endogenous Phytohormones in Pea (Pisum sativum L.). Plasma Chem Plasma Process 35, 659–676 (2015). https://doi.org/10.1007/s11090-015-9627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-015-9627-8