Abstract
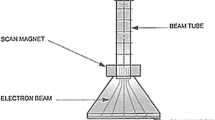
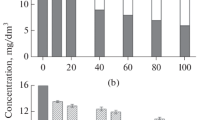
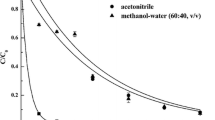
To identify the decomposition characteristics of trimethylamine (TMA) by electron beam (EB), we conducted an experiment based on process parameters, including absorbed dose (2.5–10 kGy), background gas (air, O2, N2 and He), water content (1,200, 14,300, and 27,500 ppm), initial concentration (50, 100, and 200 ppm) and reactor type (batch or continuous flow system). Air background gas showed a maximum TMA removal efficiency of 86 % at 10 kGy and that was the highest efficiency of all background gases. Energy efficiencies were higher when the absorbed dose was lower (e.g., 2.5 kGy). Decomposition efficiencies of all initial TMA concentrations were approximately >90 % at 10 kGy. Removal efficiencies increased up to 30 % as water vapor increased. As a by-product, it is observed that CH3 radical formed by EB irradiation was converted into CH4 by reaction with residual TMA, (CH3)2NH, and H. These results suggest that EB technology can be applied for TMA treatment under low concentration and high flow rate conditions.






Similar content being viewed by others
References
Kabir E, Kim KH (2010) An on-line analysis of 7 odorous volatile organic compounds in the ambient air surrounding a large industrial complex. Atmos Environ 44:3492–3502
Ding Y, Shi JY, Wu WX, Yin J, Chen YX (2007) Trimethylamine (TMA) biofiltration and transformation in biofilters. J Hazard Mater 143:341–348
US EPA (2008) Acute exposure guideline levels (AEGLs) for trimethylamine (CAS Reg. No. 75-50-3)
Chang MB, Tseng TD (1996) Gas-phase removal of H2S and NH3 with dielectric barrier discharges. J Environ Eng 122:41–46
Son YS, Kim KJ, Kim JC (2010) A review on VOCs control technology using electron beam. AJAE 4:63–71
Xia L, Huang L, Shu X, Zhang R, Dong W, Hou H (2008) Removal of ammonia from gas stream with dielectric barrier discharge plasma. J Hazard Mater 152:113–119
Kim NJ, Sugano Y, Hirai M, Shoda M (2000) Removal of a high load of ammonia gas by a marine bacterium, Vibrio alginolyticus. J Biosci Bioeng 90:410–415
Chaichanawong J, Tanthapanichakoon W, Charinpanitkul T, Eiad-ua A, Sano N, Tamon H (2005) High-temperature simultaneous removal of acetaldehyde and ammonia gases using corona discharge. Sci Technol Adv Mater 6:319–324
Tanthapanichakoon W, Charinpanitkul T, Chaiyo S, Dhattavorn N, Chaichanawoug J, Sano N, Tamon H (2004) Effect of oxygen and water vapor on the removal of styrene and ammonia from nitrogen by non-pulse corona-discharge at elevated temperatures. Chem Eng J 97:213–223
Kim JC (2002) Factors affecting aromatic VOC removal by electron beam treatment. Radiat Phys Chem 65:429–435
Son YS, Park KN, Kim JC (2010) Control factors and by-products during decomposition of butane in electron beam irradiation. Radiat Phys Chem 79:1255–1258
Hirota K, Hakoda T, Arai H, Hashimoto S (2002) Electron-beam decomposition of vaporized VOCs in air. Radiat Phys Chem 65:415–421
Son YS, Son YS, Park JH, Kim P, Kim JC (2012) Oxidation of gaseous styrene by electron beam irradiation. Radiat Phys Chem 81:686–692
Sun Y, Chmielewski AG, Bulka S, Zimek Z (2006) Influence of base gas mixture on decomposition of 1,4-dichlorobenzene in an electron beam generated plasma reactor. Plasma Chem Plasma Process 26:347–359
Chmielewski AG (2007) Industrial applications of electron beam flue gas treatment-from laboratory to the practice. Radiat Phys Chem 76:1480–1484
Mätzing H (1991) Chemical kinetics of flue gas cleaning by irradiation with electrons. In: Prigogine I, Rice SA (eds) Advances in chemical physics, vol LXXX. Wiley, New York, pp 315–402
Kim KJ, Kim JH, Son YS, Chung SG, Kim JC (2012) Advanced oxidation of aromatic VOCs using a pilot system with electron beam-catalyst coupling. Radiat Phys Chem 81:561–565
Son YS, Kim KJ, Kim JC (2010) Comparison of the decomposition characteristics of aromatic VOCs using an electron beam hybrid system. Radiat Phy Chem 79:1270–1274
Atkinson R, Pitts JN (1978) Kinetics of the reactions of O(3P) atoms with the amines CH3NH2, C2H5NH2, (CH3)2NH, and (CH3)3N over the temperature range 298–440°K. J Chem Phy 68:911–915
Tuazon EC, Atkinson R, Aschmann SM, Arey J (1994) Kinetics and products of the gas-phase reactions of O3 with amines and related compounds. Res Chem Intermed 20:303–320
Ogryzlo EA, Tang CW (1970) Quenching of oxygen (1Σg) by amines. J Am Chem Soc 92:5034–5036
Atkinson R, Perry RA, Pitts JN (1978) Rate constants for the reactions of the OH radical with (CH3)2NH, (CH3)3N, and C2H5NH2 over the temperature range 298–426°K. J Chem Phys 68:1850–1853
Das S, Schuchmann MN, Schuchmann H-P, Sonntag CV (1987) The production of the superoxide radical anion by the OH radical-induced oxidation of trimethylamine in oxygenated aqueous solution. The kinetics of the hydrolysis of (hydroxymethyl) dimethylamine. Chem Ber 120:319–323
Schlegelmilch M, Streese J, Steqmann R (2005) Odour management and treatment technologies: an overview. Waste Manag 25:928–939
Deac I, Almasan V, Palibroda N, Indrea E, Filip X, Ursu I (1996) A low energy source for production of CH3, CN and other free radical: IRMPD of di and trimethylamine molecules. Appl Surf Sci 106:223–227
Kozok PJ, Gesser H (1960) The photolysis of triethylamine, and reactions of methyl radicals with triethylamine and diethylamine. J Chem Soc (resumed) 448–452. doi:10.1039/JR9600000448
Bamford CH (1939) A study of the photolysis of organic nitrogen compounds. Part I. Dimethyl- and diethyl-nitrosoamines. J Chem Soc (resumed) 12–17. doi:10.1039/JR9390000012
Bamford CH (1939) A study of the photolysis of organic nitrogen compounds. Part II. Aliphatic amines. J Chem Soc (resumed) 17–26. doi:10.1039/JR9390000017
Gesser H, Mullhaupt JT, Griffiths JE (1957) The photolysis of trimethylamine. J Am Chem Soc 79:4834–4836
Seetula J, Blomqvist K, Kalliorinne K, Koskikallio J (1985) Kinetics of reactions between CH3, CH3CO and (CH3)2N radicals produced by flash-photolysis of N, N-dimethylacetamide in gas-phase. Finn Chem Lett 69:139–140
Seetula J, Blomquist K, Kalliorinne K, Koskikallio J (1986) Kinetics of radical reactions between methyl, acetyl and dimethylamino radicals formed in the flash photolysis of N, N-dimethylacetamide in the gas phase. Acta Chem Scand 40:658–663
Lindley CRC, Calvert JG, Shaw JH (1979) Rate studies of the reactions of the (CH3)2N Radical with O2, NO, and NO2. Chem Phys Lett 67:57–62
Gray P, Jones A, Thynne JCJ (1965) Kinetics and sites of methyl radical attack on dimethylamine and deuterated dimethylamine. Trans Faraday Soc 61:474–483
Tsang W (1989) Rate constants for the decomposition and formation of simple alkanes over extended temperature and pressure ranges. Combust Flame 78:71–86
Edwards DA, Kerr JA, Lloyd AC, Trotman-Dickenson AF et al (1966) Hydrogen-abstraction reactions by methyl radicals from nitrogen-containing compounds. J Chem Soc A 621–622. doi:10.1039/J19660000621
Anderson KM, Ou D, Wu YB, Jajeh A, Harris JE (1999) Induction of type 1 programmed cell death in U937 cells by the antioxidant, butylated hydroxy-toluene or the free radical spin trap, NTBN. Leuk Res 23:665–673
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012R1A6A3A03039668). This study was also supported by the Korean Ministry of Environment as part of “The Eco-Technopia 21 Project”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Son, YS., Kim, P., Park, J.H. et al. Decomposition of Trimethylamine by an Electron Beam. Plasma Chem Plasma Process 33, 1099–1109 (2013). https://doi.org/10.1007/s11090-013-9479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-013-9479-z