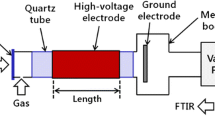
Abstract
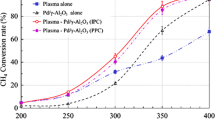
The destruction of methane by a non‐thermal plasma in atmospheric pressure gas streams of nitrogen with variable amounts of added oxygen has been investigated. The identities and concentrations of the end‐products are determined by on‐line FTIR spectroscopy and the plasma chemistry is interpreted using kinetic modelling. For a deposited energy of 118 kJ m−3, the destruction is 12% in nitrogen decreasing monotonically to 5% in air. The major end‐products are HCN and NH3 in nitrogen and CO, CO2, N2O, NO and NO2 for gas streams containing oxygen. The chemistry in pure nitrogen is predominantly due to reactions of electronically‐excited nitrogen atoms, N(2D). The addition of oxygen converts the excited state nitrogen into nitrogen oxides reducing the methane destruction which then arises by O and OH reactions yielding CO and, to a lesser extent, CO2. The modelling correctly predicts the magnitude of the methane destruction as a function of added oxygen and the concentrations of the end‐products for processing in both nitrogen and air.
Similar content being viewed by others
References
1. L. M. Zhou, B. Xue, U. Kogelschatz, and B. Eliasson, Plasma Chem. Plasma Proc. 18, 375 (1998).
2. M. Okumoto, B. S. Rajanianth, S. Katsura, and A. Mizuno, IEEE Trans. Ind. Appl. 34, 940 (1998).
3. M. Okumoto, Z. Su, S. Katsura, and A. Mizuno, IEEE Trans. Ind. Appl. 35, 1205 (1999).
4. S. L. Yao, T. Takemoto, F. Ouyang, A. Nakayama, E. Suzuki, A. Mizuno, and M. Okumoto, Energy Fuels 14, 459 (2000).
5. H. Matsumoto, S. Tanabe, K. Okitsu, Y. Hayashi, and S. L. Suib, J. Phys. Chem. A 105, 5304 (2001).
6. L. Bromberg, D. R. Cohn, A. Rabinovich, C. O’Brien, and S. Hochgreb, Energy Fuels 12, 11 (1998).
7. L. M. Zhou, B. Xue, U. Kogelschatz, and B. Eliasson, Energy Fuels 12, 1191 (1998).
8. B. Eliasson, C. Liu, and U. Kogelschatz, Ind. Eng. Chem. Res. 39, 1221 (2000).
9. M. Kraus, B. Eliasson, U. Kogelschatz, and A. Wokaun, Phys. Chem. Chem. Phys. 3, 294 (2001).
10. K. Zhang, U. Kogelschatz, and B. Eliasson, Energy Fuels 15, 395 (2001).
11. S. Kado, Y. Sekine, and K. Fujimoto, Chem. Commun., 2485 (1999).
12. C. Marún, S. L. Suib, M. Dery, J. B. Harrison, and K. Kablaoui, J. Phys. Chem. 100, 17866 (1996).
13. C. Marún, L. D. Conde, and S. L. Suib, J. Phys. Chem. A 103, 4332 (1999).
14. T. Fujii and M. Kareev, J. Appl. Phys. 89, 2543 (2001).
15. D. Liu, T. Ma, S. Yu, Y. Xu, and X. Yang, J. Phys. D: Appl. Phys. 34, 1651 (2001).
16. M. N. R. Ashfold, P. W. May, J. R. Petherbridge, K. N. Rosser, J. A. Smith, Y. A. Mankelevich, and N. V. Suetin, Phys. Chem. Chem. Phys. 3, 3471 (2001).
17. D. E. Tevault, Plasma Chem. Plasma Proc. 5, 369 (1985).
18. C. D. Pintassilgo, J. Loureiro, G. Cernogora, and M. Touzeau, Plasma Sources Sci. Technol. 8, 463 (1999).
19. M. Kareev, M. Sablier, and T. Fujii, J. Phys. Chem. A 104, 7218 (2000).
20. J.-C. Legrand, A.-M. Diamy, R. Hrach, and V. Hrachová, Vacuum 48, 671 (1997).
21. J.-C. Legrand, A.-M. Diamy, R. Hrach, and V. Hrachová, Vacuum 50, 491 (1998).
22. J.-C. Legrand, A.-M. Diamy, R. Hrach, and V. Hrachová, Vacuum 52, 27 (1999).
23. J. Röpcke, L. Mechold, M. Käning, W. Y. Fan, and P. B. Davies, Plasma Chem. Plasma Proc. 19, 395 (1999).
24. W. Y. Fan, P. F. Knewstubb, M. Käning, L. Mechold, J. Röpcke, and P. B. Davies, J. Phys. Chem. A 103, 4118 (1999).
25. A. Ogata, K. Mizuno, S. Kushiyama, and T. Yamamoto, Plasma Chem. Plasma Proc. 18, 363 (1998).
26. C. Fitzsimmons, F. Ismail, J. C. Whitehead, and J. J. Wilman, J. Phys. Chem. A 104, 6032 (2000).
27. P. L. Hanst and S. T. Hanst, Infrared Spectra for Quantitative Analysis of Gases, Infrared Analysis, Anaheim, CA (1996).
28. T. Yamamoto, J. Haz. Mat. B67, 165 (1999).
29. P. Jemmer, Math. Comp. Mod. 30, 61 (1999).
30. R. J. Kee, F. M. Rupley, and J. A. Miller, Chemkin-II: A Fortran Chemical Kinetics Package for the Analysis of Gas Phase Chemical Kinetics, Sandia National Laboratory (1991).
31. A. C. Gentile and M. J. Kushner, J. Appl. Phys. 78, 2074 (1995).
32. A. C. Gentile and M. J. Kushner, J. Appl. Phys. 78, 2977 (1995).
33. W. L. Morgan and B. M. Penetrante, Comp. Phys. Comm. 58, 127 (1990).
34. T. Nakano, H. Toyoda, and H. Sugai, Jap. J. Appl. Phys. 30, 2908 (1991).
35. C. Fitzsimmons, J. T. Shawcross, and J. C. Whitehead, J. Phys. D: Appl. Phys. 32, 1136 (1999).
36. J. Li, W. Sun, B. Pashaie, and S. K. Dhali, IEEE Trans. Plasma Sci. 23, 672 (1995).
37. M. F. Golde, Int. J. Chem. Kin. 20, 75 (1988).
38. K. Schofield, J. Phys. Chem. Ref. Data 8, 723 (1979).
39. G. Marsden, F. L. Nesbitt, D. F. Nava, W. A. Payne, and L. J. Stief, J. Phys. Chem. 93, 5769 (1989).
40. A. Ogata, K. Yamanouchi, K. Mizuno, S. Kushiyama, and T. Yamamoto, IEEE Trans. Ind. Appl. 35, 1289 (1999).
41. W. G. Mallard, F. Westley, J. T. Herron, R. F. Hampson, and D. H. Frizzell, NIST Chemical Kinetics Database; Windows Version 2Q98 edition; U.S. Department of Commerce, National Institute of Standards and Technology, Gaithersburg (1998).
42. H. R. Snyder and G. K. Anderson, IEEE Trans. Plasma Sci. 26, 1695 (1998).
43. A. R. Martin, J. T. Shawcross, and J. C. Whitehead, J. Phys. D: Appl. Phys., 37, 42 (2004).
44. J. Hoard, T. J. Wallington, J. C. Ball, M. D. Hurley, and K. Wodzisz, Env. Sci. Tech. 33, 3427 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pringle, K., Whitehead, J., Wilman, J. et al. The Chemistry of Methane Remediation by a Non‐thermal Atmospheric Pressure Plasma. Plasma Chem Plasma Process 24, 421–434 (2004). https://doi.org/10.1007/s11090-004-2277-x
Issue Date:
DOI: https://doi.org/10.1007/s11090-004-2277-x