Abstract
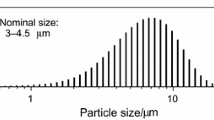
The kinetics of complete oxidation of different samples of zinc powder by air has been investigated by thermogravimetric measurements under isothermal conditions in the range 973–1,173 K. Particles size was in the 63–80 μm range. We succeeded in carrying out the full oxidation of the powders far above the zinc-metal melting point (692.6 K). This phenomenon is linked to the presence of a thin ZnO layer which confines the liquid metal during the oxidation process. Two kinetics models have been verified. The apparent activation energies obtained from the Arrhenius law were 128 kJ mol−1 and 129 kJ mol−1, respectively.






Similar content being viewed by others
References
D. Clarke, Journal of the American Ceramic Society 82(3), 485 (1999).
R. Metz, H. Delalu, J. R. Vignalou, N. Achard, and M. Elkhatib, Materials Chemistry and Physics 63, 157 (2000).
R. Metz, C. Machado, M. Houabes, M. Elkhatib, and M. Hassanzadeh, Journal of Materials Processing Technology 195(1–3), 248 (2008).
J. Clayton, H. Takamura, R. Metz, H. Tuller, and B. Wuensch, Journal of Electroceramics 7(2), 113 (2001).
J. Benard, L’oxydation des métaux, processus fondamentaux (I) (Gauthier-Villars, Paris, 1962).
C. Tuck, M. Whitehead, and R. Smallman, Corrosion Science 21, 333 (1981).
V. I. Dybkov, Growth Kinetics of Chemical Compound Layers (Cambridge International Science Publishing, Cambridge, England, 1998).
H. Delalu, J. R. Vignalou, M. Elkhatib, and R. Metz, Solid State Sciences 2(2), 229 (2000).
C. Machado, S. Aidel, M. Elkhatib, H. Delalu, and R. Metz, Solid State Ionics 149, 147 (2002).
M. L. Zheludkevich, A. G. Gusakov, A. G. Voropaev, A. A. Vecher, E. N. Kozyrski, and S. A. Raspopov, Oxidation of Metals 61, 39 (2004).
A. Rai, K. Park, L. Zhou, and M. R. Zachariah, Combustion Theory and Modelling 10, 843 (2006).
A. G. Gusakov, A. G. Voropaev, M. L. Zheludkevich, A. A. Vecher, and S. A. Raspopov, Physical Chemistry Chemical Physics 1, 5311 (1999).
Acknowledgement
We would like to express our gratitude to Socrates-Erasmus Student Mobility Program since one of us obtained a grant between Jaén University (Spain) and Lyon1 University (France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
García, J.F., Sánchez, S. & Metz, R. Complete Oxidation of Zinc Powder. Validation of Kinetics Models. Oxid Met 69, 317–325 (2008). https://doi.org/10.1007/s11085-008-9099-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-008-9099-9