Abstract
The justification for a less alkaline primordial ocean (than present) is briefly reviewed, along with constraints on aqueous phosphate under such conditions. Based on the assumption that CaHPO4 dihydrate determined the availability of phosphorus species, we have carried out laboratory simulations to determine equilibrium concentrations as a function of pH (in PIPES buffer) with added NaCl and CaCl2. Consistent with expectations, solubility declines with higher pH and [CaCl2], but increases only slightly with [NaCl]. Significantly, PIPES shows no specific effect on the dissolution beyond its influence on pH and ionic strength. Data are also presented on the synthesis of pyrophosphate from the NaOCN/CaHPO4·2H2O system, which could have provided a source of this phosphate anhydride on the early Earth.
Similar content being viewed by others
Introduction
The low solubility of phosphate (≤3 μM) in marine environments has long been recognized as an obstacle in the elucidation of prebiotic pathways to phosphate esters, if similar constraints operated in the past (Gulick 1955; Keefe and Miller 1995). Two possible strategies for enhancing aqueous concentrations have included the use of Ca2+-complexing agents (such as oxalate) and a lower pH relative to the modern value near 8.3 (Schwartz 1971). Indeed, Gedulin and Arrhenius (1994) made the remarkable observation that in the pH range of 6–7.5, brushite (CaHPO4·2H2O) is the (heat-annealed) mineral phase that crystallizes during the controlled introduction of phosphate into a modified, sulfate-free seawater medium, and constitutes the only solid species formed between pH 6 and 7; this material is also substituted with several mole-percent magnesium, thus lowering the solubility toward whitlockite, Ca18Mg2H2(PO4)14. Although considerable uncertainty exists regarding the composition of early ocean, aqueous acidity would have been sensitive to the higher CO2 that is associated with the Archean atmosphere (Walker 1983). Whether partial pressures ever reached 1,000-times the present value during the initial outgassing, Grotzinger and Kasting (1993) have shown that a pCO2 of 0.03 would bring the marine pH within the stability range of brushite. This level of carbon dioxide is consistent with the upper limit of 0.04 atm. at 2.75 Gya proposed by Rye et al. (1995). CaHPO4·2H2O therefore merits closer scrutiny as a potential source of prebiotic phosphate compounds.
The central advantage of brushite for prebiotic simulations is its higher solubility compared to the apatite phases (Jaynes et al. 1999). While the dissolution of the former has been studied in the context of agricultural use (Bennett and Adams 1976), we have sought to measure phosphate concentrations as a function of pH, salinity and added CaCl2 under a wider range of conditions using an aqueous, magnesium-free medium. These equilibration experiments have been carried out using a commercial form of CaHPO4·2H2O (Aldrich) that is compositionally similar to authentic brushite, except for the absence of magnesium-substitution. Remarkably, magnesium-free brushite is known to grow on human skeletons that have been left in crypts and stone coffins (Piepenbrink 1984)!
A related question is whether brushite can react with cyanate to give pyrophosphate. Miller and Parris (1964) reported an analogous transformation using the phosphate mineral, hydroxyapatite, which is not regarded as prebiotic because below pH 8.5 apatite forms only through an organism-mediated process. CaHPO4·2H2O has previously been investigated in the synthesis of nucleoside triphosphates from the corresponding diphosphates in the presence of cyanate as a condensing agent (Yamagata 1999), but there have been no studies that address the formation of inorganic pyrophosphate.
Experimental Section
Materials
All chemicals were reagent grade. Sodium chloride, sodium cyanate, sulfuric acid, NaOH (solid), NaH2PO4 and CaHPO4·2H2O were obtained from Aldrich Chemical Co., l-ascorbic acid and piperazine-N,N′-bis(2-ethanesulfonic acid) were purchased from Sigma Chemical, calcium chloride from J. T. Baker, ammonium molybdate and hydrochloric acid (37%) from Mallinckrodt, aqueous sodium hydroxide (50%) and tetrasodium pyrophosphate (decahydrate) from Fisher, and trisodium naphthalenetrisulfonate from TCI-America. The enzymatic “pyrophosphate reagent” (P-7275) was also purchased from Sigma. Glassware was washed in phosphate-free detergent (lauryl sulfate) from Sigma, and deionized water was used to prepare all solutions.
Methods
Equilibration experiments were carried out in quadruplicate at ambient temperature, maintained at 21 + 1°C over a 15-day period. A typical dissolution mixture consisted of 0.100 g CaHPO4·2H2O in a 20 ml glass vial with 5.00 ml of a mixture containing NaCl (0.25–1.00 M), CaCl2 (0.01–0.04 M) and PIPES (0.05–0.4 M) adjusted with dilute NaOH to the desired pH before addition. A plastic bead (1 cm diameter) was added before sealing to facilitate agitation, and the suspension was placed on a mechanical shaker. At intervals of three to four days, 200–400 μl was withdrawn with a micropipettor and sedimented using a Fisher MicroV Centrifuge for 10 min at 7,000 r.p.m. An aliquot of 40 μl was removed from the clear supernatant for subsequent analysis of soluble phosphate by a spectrophotometric method described below.
Reactions (with three to four replicates) were carried out by adding 50 mg CaHPO4·2H2O to a screw-cap test tube (10 × 1.3 cm) to which was added 2.00 ml of aqueous NaOCN (5–20 mM), adjusted to the appropriate pH with HCl. After mixing on a “Vortexer” (VWR), the tubes were incubated for two weeks at 60°C in a VWR dry-block, and then centrifuged with a Clay‐Adams centrifuge after cooling to room temperature. The pH of the supernatant liquid was recorded using a Fisher Accumet meter (Model 10) equipped with a pencil-thin electrode.
Phosphate analysis
Phosphate was assayed by a modification of the procedure of Chen et al. (1956): 40 μl of the supernatant was added to 3.96 ml of a reagent mixture containing 0.32 M H2SO4, 0.5% (w/v) ascorbic acid and 0.13% (w/v) in an 8 ml screw-cap test tube. After mixing, the capped tube was incubated at 37°C for 1 h, and the absorbance at 810 nm was measured in a 1 cm cuvette (with water as a reference) using a Perkin‐Elmer λ-6 UV‐VIS Spectrophotometer. Standards were prepared by adding 60–300 nmol of a stock solution of 0.0300 M NaH2PO4 to the mixture of molybdate/ascorbic acid/sulfuric acid under the same conditions of volume and concentration. A pair of blanks with only water added were averaged to correct for the background absorption at 810 nm; phosphate concentrations for the unknowns were calculated from a least-squares equation obtained from the plot of five standards (not shown). For each set of four replicate analyses, the phosphate concentrations were expressed as the mean along with the 95% confidence limits obtained by using the appropriate Student t-value. The one exception to this procedure was during the 15-day assay of the dissolution in 1.0 M NaCl (Table II), where only three data were used.
Pyrophosphate analysis
A liquid chromatography method described by Shamsi and Danielson (1993) was employed, in which analyte concentrations are calculated from the decrease in UV absorption of the mobile phase (indirect photometric detection). The assay was carried out with a Buck BLC-20 system equipped with a Hamilton PRP-X100 column (15 × 0.4 cm) at a flow of 2 ml/min and a wavelength of 280 nm. A mobile phase of 1.5 × 10−4 M naphthalenetrisulfonate in 5% (v/v) acetonitrile and an injection volume of 20 μl were used in all cases. The presence of pyrophosphate in the reactions was also confirmed by the oxidation of nicotinamide adenine dinucleotide in the enzyme mixture from Sigma.
Results and Discussion
A primary objective of this study was to explore the effect of pH on phosphate solubility using CaHPO4·2H2O as the source. Since “manual” adjustment by addition of acid did not seem practical for a large number of replicate samples, we chose to use 1,4-piperazinebis(ethanesulfonic acid) as a buffer. Although PIPES itself was unlikely to have been present on the early Earth, it has a pK a (25°C) near 6.8 that allows effective control over the pH in our studies. Moreover, because of its poor ability to bond to metals such as Ca2+ (Good et al. 1966), we believed that PIPES could regulate the pH without dramatically affecting the solubility of phosphate.
The direct effect of PIPES was tested as shown in Table I: total phosphate in the supernatant solution was assayed after equilibration over 15 days at several different concentrations of buffer, from 0.05 to 0.4 M. These mixtures also contained 0.50 M NaCl and 0.010 M CaCl2, in order to approximate the average concentrations typical of the modern ocean (Krauskopf and Bird 1995). Although the pH increased slightly from that of the initial buffer, the final values span a relatively narrow range (<0.06 within replicate sets), and the associated phosphate concentrations do not show a strong dependence on [PIPES] as would be expected if there were a specific interaction with the CaHPO4·2H2O.
The effect of [NaCl] on the solubility of CaHPO4·2H2O is also of interest, especially since Holland (1984) has suggested that the chloride concentration in the early ocean might have been slightly higher: 0.7 mol kg−1 (vs. about 0.53 mol kg−1 today). Although salinity levels are largely uncontrained in the Archean, Knauth (2005) has estimated that they may have been up to twice the current concentrations (based on revised halite deposits). As shown in Table II, we find that there is a linear increase in soluble phosphate associated with higher [NaCl] – using the results from the 15 day equilibration, [Pi] = (1.896 × 10−3) [NaCl] + (3.025 × 10−3 M) with R = 0.994. A doubling in the ionic strength causes only about a 20% rise in phosphate, and an even smaller effect has been reported with KCl (Bennett and Adams 1976); however, this influence is consistent with the very slight increases observed with higher [PIPES] in Table I. Thus, if phosphate availability in the early seas were determined by CaHPO4·2H2O, it is unlikely that a higher salinity would have a significant impact (unless it was associated with Mg2+ salts that could cause substitution in the brushite mineral).
Table II also includes phosphate concentrations measured after 11 and 15 days of mixing. As described in the Experimental Section, we analyzed the supernatant at three to four day intervals over a 15 day period, in order to be confident that equilibrium had been reached; a comparison of the data at these times indicated no statistically significant differences, so that this interval was used for other assays (Table I and Figure 1). Equilibration over 15 days is similar to the two-week period employed by Bennett and Adams (1976).
Effect of Ca2+ on phosphate solubility. Figure 1 Shows the correlation between final pH and soluble phosphate for three different concentrations of added CaCl2 (10, 20, and 40 mM). All equilibration mixtures contained 0.50 M NaCl and 0.1 M PIPES, and were assayed after 15 days at 21°C. Error bars represent the 95% confidence limits for replicate samples.
The effect of pH and added CaCl2 is shown in Figure 1. A medium containing 0.5 M NaCl and 0.1 M PIPES was allowed to equilibrate for 15 days at ambient temperature (21°C) before analysis. The addition of calcium ions was relevant, because Krauskopf and Bird (1995) have pointed out that as pCO2 increases and the pH of the early ocean likely became more acidic, the enhanced solubility of calcium and magnesium carbonates would result in higher Ca2+ and Mg2+ concentrations. Not surprisingly, we find that calcium depresses the concentration of soluble phosphate, in a manner that is roughly proportional to added CaCl2: the lowest value was in the presence of 40 mM calcium, where a concentration of 0.70 mM at pH 6.8 was recorded. By comparison, 20 mM CaCl2 “raised” the [Pi] to 1.48 mM (pH 6.7) and 10 mM CaCl2 caused a phosphate concentration of 3.17 mM (pH 6.7). This effect is a natural consequence of Le Chatelier’s Principle, shifting the equilibrium toward solid CaHPO4·2H2O as exogenous calcium is introduced:
(Bennett and Adams 1976)
However, this solubility product (extrapolated to zero ionic strength) does not take into account the formation of ion pairs (CaHPO4 0 and CaH2PO4 +) in solution, which slightly lessens the precipitation of phosphate by added CaCl2 (McDowell et al. 1970). It should be noted that a higher concentration of aqueous Mg2+ would also have suppressed brushite solubility through the replacement of calcium ions.
The relationship between pH and solubility was investigated for each of the three levels of added CaCl2. As noted above, a buffer of 0.10 M PIPES (adjusted with NaOH to an initial pH of 6.0, 6.5, 7.0 or 7.5) was used to control the acidity; after the 15 day equilibration the final pH was about 0.2–0.3 units lower. In the presence of 10 mM CaCl2, the concentration of soluble phosphate doubled from 2.82 mM (pH 7.3) to 5.63 mM (pH 5.8). Although we studied only a small number of different acidities, the curves over this range suggest an exponential increase in solubility as the pH is lowered, due to the conversion of HOPO3 2− to (HO)2PO2 −:
(Krauskopf and Bird 1995)
As [H3O+] becomes higher, a greater proportion of phosphate is present as the monoanion, which does not precipitate as readily as the dianion in the presence of calcium ions.
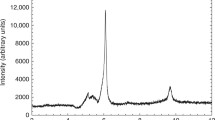
Table III shows the yields of pyrophosphate, analyzed by HPLC after two weeks at 60°C, which is within the estimated range of Archean ocean temperatures published by Knauth (2005). These data represent the actual concentrations in the supernatant phase after cooling to room temperature, without any specific treatment to extract the product. (A few of the chromatograms did show a peak for the inorganic triphosphate, but the results were not reproducible and are therefore not included.) The pH drops as the reaction reaches completion, presumably a consequence of the hydrolysis of cyanic acid (initially to carbamic acid), but no attempt was made to buffer the solutions beyond adjusting the initial pH (Amell 1956). The yields of pyrophosphate represent averages of three to four measurements, and are based on the assumption that one molecule of cyanate would produce one pyrophosphate.
The highest yield (4.5 + 1.4%) was observed starting with 5 mM NaOCN, initially at pH 7. Surprisingly, the percentage declines (by half) when one compares the results starting with 10 mM cyanate at the same pHi, and drops by the same amount when the concentration is doubled to 20 mM cyanate. However, it is known (Vieyra et al. 1995) that pyrophosphate can also suppress its synthesis from the intermediate carbamoyl phosphate in the presence of calcium phosphate precipitates (presumably due to competitive binding to catalytic sites), and the limiting concentration of pyrophosphate (0.2 mM) may reflect such a product inhibition of the catalysis. Only a narrow range of acidities was examined (6.5 < pHi < 7.5) to keep within the stability field of brushite, but these limited data nevertheless do suggest that the highest concentrations of pyrophosphate are obtained at a near-neutral starting pH.
Relevance to Prebiotic Chemistry
These results provide a very tentative estimate of phosphate concentrations in the primordial ocean at 21°C, if they were controlled by equilibration with CaHPO4·2H2O: dependent on pH and [Ca2+], soluble phosphate may have been 102–103 times higher than the maxima observed today in deep ocean environments where abiotic processes (dissolution of apatite from skeletons and hydrolysis of biogenic organics) are believed to dominate (Atlas and Pytkowicz 1977). If these concentrations are in any sense representative of Archean seawater, they provide a more pragmatic basis for the design of prebiotic phosphorylation reactions, which are extremely difficult to study if one is limited to micromolar levels of aqueous phosphate. Local environments nevertheless may have played an important role in enhancing rates of prebiotic processes, as in the interlayer space of “anti-clays” that can readily adsorb and phosphorylate organic anions (Kolb et al. 1997). While alternative scenarios, such as phosphonic acids as analogs, have been proposed in order to circumvent the solubility problem of phosphate (De Graaf et al. 1997), we should also consider the possibility that a neutral or slightly acidic pH in marine environments allowed [Pi] to approach the millimolar level.
An important factor in applying these results is that our reaction system was chosen to ignore the effects of the magnesium ion. Modern seawater is distinguished by a molar ratio of about 5:1 (Henderson 1982) for the relative amounts of Mg2+ (0.05 M) and Ca2+ (0.01 M). While the composition of the Archean ocean is poorly constrained, Silurian seawater (Brennan and Lowenstein 2002) may have contained similar levels of magnesium, but also a higher concentration of calcium (or a ratio of approximately 1.4:1). As noted above, Mg2+ can replace Ca2+ within the brushite structure, thereby diminishing the phosphate solubility. Such concentrations of aqueous magnesium (and ammonium ion) may also have favored the synthesis of another reactive phosphate species, known as struvite (MgCaPO4·6H2O), as shown by Handschuh and Orgel (1973). On the other hand, magnesium has virtually no effect on the rate of brushite formation (Salimi et al. 1985).
Pyrophosphate was suggested by Lipmann as an activated form of phosphate (Lipmann 1965), and indeed it can phosphorylate adenosine in the presence of apatite (Neuman et al. 1970). (While not considered a prebiotic mineral for the reasons noted above, such an observation does demonstrate the power of surface catalysis from a chemical perspective.) Cyanoguanidine (a dimer of cyanamide) gave a 1.8% conversion to pyrophosphate in the presence of kaolinite, but only under acidic (pH < 2) conditions and 0.1 M concentrations of both reactants (Steinman et al. 1965). Condensing agents investigated by Keefe and Miller (1996) that were effective in the synthesis of pyrophosphate include maleic anhydride, pantoyl lactone and ammonium formate, but these are noteworthy for the use of high concentrations (0.25 M) and high temperatures (100°C) that render the syntheses implausible; of particular interest was a mixture of thiocyanate, hydrogen peroxide and phosphate, which gave efficient conversion to pyrophosphate under milder conditions, but as Keefe and Miller noted, the yields are too sensitive to the ratio of the reagents to regard it as “robust.” Thioesters such as N, S-diacetylcysteamine are compelling because of their relevance to modern metabolism, but the prebiotic importance to the formation of pyrophosphate is diminished by the high concentrations: 0.1–0.2 M for the thioester, 0.2–0.4 M for the imidazole catalyst and 0.08–0.4 M for inorganic phosphate (Weber 1981, 1982). Recently, the hydrolysis of iron phosphide has been investigated as a model for the meteoritic delivery of phosphorus species, but the final concentration of pyrophosphate reported for a degassed, aqueous phase is an order of magnitude lower than that obtained in our reaction system (Pasek and Lauretta 2005). We therefore believe that the synthesis reported here is more prebiotically significant than these previous studies, especially because of the low concentration of cyanate (down to 5 mM) and the reliance on CaHPO4 dihydrate as the sole source of phosphate at near-neutral pH.
Thermal syntheses of pyrophosphate has been reported, including the heating of Ca(H2PO4)2·H2O and other dihydrogen phosphates at 160°C (Rabinowitz et al. 1968), but these acidic species are not found as minerals in nature today (Keefe and Miller 1995). Of greater relevance is the observation that pyrophosphate (and oligophosphates up to the octamer or longer depending on reaction time and water removal) can be formed from heating brushite in the dry state at 500°C, and more slowly at lower temperatures (Gedulin and Arrhenius 1994). Such conditions would (likely) have been limited to volcanic environments, which must have been even more widespread and active on the early Earth, due to juvenile radioactive decay (Arrhenius et al. 1997; Schwartz and Henderson-Sellers 1983).
The condensing agent, cyanate, has been synthesized under a variety of experimental conditions, including from CO2/H2/N2 atmospheres subjected to electrical discharge (Yamagata and Mohri 1982) as well as UV photolysis of CO/NH3 (Ferris et al. 1974) and HCN/H2O/NH3 mixtures (Garakines et al. 2004). The first of these routes, which the authors observed was strongly dependent upon the presence of molecular hydrogen (mixing ratios of 0.14–0.50), has acquired new relevance in view of revised estimates of H2 escape that favor a more hydrogen-rich atmosphere for the early Earth (Tian et al. 2005). While the importance of heterogeneous catalysis in the cyanate-mediated synthesis of pyrophosphate has been noted by previous authors (Vieyra et al. 1995; Beck and Orgel 1965), such studies have employed calcium phosphate precipitates that are not as well characterized as the CaHPO4·2H2O used in this work. Similarly, the observation that iron minerals promote pyrophosphate formation at near-neutral pH is a significant finding, but that investigation was restricted to highly activated starting materials, such as phosphoenolpyruvate and acetyl phosphate (De Zwart et al. 2004).
Conclusion
Gedulin and Arrhenius (1994) found that apatite formed from seawater exclusively at high pH (>8.5) or by organismic mediation at lower values, while whitlockite and Mg3(PO4)3·5H2O crystallized in the range of pH 7–9; as noted in the introduction, most likely Mg2+-substituted brushite was the only mineral phase found upon thermal annealing of the nanocrystalline phosphate gel from slightly acidic seawater (pH 6–7). A neutral or mildly acidic ocean is consistent with elevated CO2 levels that likely prevailed in the early atmosphere, so that concentrations of soluble phosphate were probably much higher compared to the deep ocean regions today, where micromolar amounts represent the upper limit.
Our equilibrations employed a commercial form of CaHPO4·2H2O, which differs from true brushite in that the former is an amorphous powder and obviously a magnesium-free analytical reagent. However, we believe that these results reflect the actual solubility, since both the dihydrate and the anhydrous form (monetite) have nearly identical K sps (suggesting that solubility is dictated by the ratio of Ca2+/HPO3 2− and even more by the Mg/Ca ratio rather than the mineral phase). Indeed, monetite probably goes into solution via surface hydration to brushite. Furthermore, the dihydrate occurs only as the brushite form (Jaynes et al. 1999), which in aquatic environments undergoes substitution by Mg2+ as noted above.
Our data indicate that the solubility of CaHPO4·2H2O would be sensitive to pH and total [Ca2+], but NaCl has little effect. Even if the concentration of aqueous calcium were several times higher than in the modern ocean, these data suggest that dissolved phosphate would have approached or exceeded the millimolar level, if the effects of magnesium incorporation are ignored. Conditions that govern the structure of brushite in marine environments merit additional study, but these data are intended as a coarse estimate of equilibrium concentrations of phosphate that might have existed early in the history of the Earth, when elevated CO2 and lower pH may have dominated the chemistry of seawater.
Finally, we have reported the first example of the cyanate-mediated synthesis of pyrophosphate from CaHPO4·2H2O. It is encouraging that this heterogeneous system can provide a source of phosphate for the reaction, especially at cyanate concentrations that are relatively low (5–20 mM). If brushite were the favored form of phosphate in marine sediments on the early Earth, then it may have undergone condensation to pyrophosphate. Future work will address the ability of cyanate to promote the synthesis of pyrophosphate and longer chains within the interlayer region of layered double hydroxides.
References
Amell AR (1956) Kinetics of the hydrolysis of cyanic acid. J Amer Chem Soc 78:6234–6238
Arrhenius G, Sales B, Mojzsis S, Lee T (1997) Entropy and charge in molecular evolution – the case of phosphate. J Theor Biol 187:503–522
Atlas E, Pytkowicz RM (1977) Solubility behavior of apatites in seawater. Limnol Oceanogr 22:290–300
Beck A, Orgel LE (1965) The formation of condensed phosphate in aqueous solution. Proc Nat Acad Sci USA 54:664–667
Bennett AC, Adams F (1976) Solubility and solubility product of dicalcium phosphate dihydrate in aqueous solutions and soil solutions. Soil Sci Soc Amer J 40:39–42
Brennan ST, Lowenstein TK (2002) The major-ion composition of Silurian seawater. Geochim et Cosmochim Acta 66:2683–2700
Chen PS Jr, Toribara TY, Warner H (1956) Microdetermination of phosphorus. Anal Chem 28:1756–1758
De Graaf RM, Visscher J, Schwartz AW (1997) Reactive phosphonic acids as prebiotic carriers of phosphorus. J Mol Evol 44:237–241
De Zwart II, Meade SJ, Pratt AJ (2004). Biomimetic phosphoryl transfer catalyzed by iron(II)-mineral precipitates. Geochim Cosmochim Acta 68:4093–4098
Ferris JP, Williams EA, Nicodem DE, Hubbard JS, Voecks GE (1974). Photolysis of CO‐NH3 mixtures and the Martian atmosphere. Nature 249:437–439
Garakines PA, Moore MH, Hudson RL (2004) Ultraviolet photolysis and proton irradiation of astrophyical ice analogs containing hydrogen cyanide. Icarus 170:202–213
Gedulin B, Arrhenius G (1994) Sources and geochemical evolution of RNA precursor molecules – the role of phosphate. In: Bengston S (ed). Early life on earth-nobel symposium 84. Columbia University Press, New York, pp91–106
Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh RMM (1966) Hydrogen ion buffers for biological resource. Biochem 5:467–477
Grotzinger JP, Kasting JF (1993) New constraints on precambrian ocean composition. J Geol 101:235–243
Gulick A (1955) Phosphorus as a factor in the origin of life. Amer Sci 43:479–489
Handschuh GJ, Orgel LE (1973) Struvite and prebiotic phosphorylation. Science 179:483–487
Henderson P (1982). Inorganic geochemistry. Pergamon, Oxford, UK, pp278–286
Holland HD (1984) The chemical evolution of the atmosphere and oceans. Princeton University Press, Princeton, New Jersey, p110
Jaynes WF, Moore PA Jr, Miller DM (1999) Solubility and ion activity of calcium phosphate minerals. J Environ Qual 28:530–536
Keefe AD, Miller SL (1995) Are polyphosphates or phosphate esters prebiotic reagents? J Mol Evol 41:693–702
Keefe AD, Miller SL (1996) Potentially prebiotic syntheses of condensed phosphates. Orig Life Evol Biosph 26:15–25
Knauth LP (2005) Temperature and salinity history of the Precambrian ocean: implication for the course of microbial evolution. Paleogeog Paleoclim Paleoecol 219:53–69
Kolb V, Zhang S, Xu Y, Arrhenius G (1997) Mineral-induced phosphorylation of glycolate ion – a metaphor in chemical evolution. Orig Life Evol Biosph 27:485–503
Krauskopf KB, Bird DK (1995) Introduction to geochemistry. McGraw-Hill, New York, pp578–600
Lipmann F (1965). Projecting backward from the present stage of evolution of biosynthesis. In: Fox SW (ed) The origins of prebiological systems and their molecular matrices. Academic, New York, pp259–280
McDowell H, Brown WE, Sutter JR (1970) Solubility study of calcium hydrogen phosphate: ion-pair formation. Inorg Chem 10:1638–1643
Miller SL, Parris M (1964) Synthesis of pyrophosphate under primitive Earth conditions. Nature 204:1248–1250
Neuman MW, Neuman WF, Lane K (1970) On the possible role of crystals in the origins of life (III): the phosphorylation of adenosine to AMP by apatite. Curr Mod Biol 3:253–259
Pasek MA, Lauretta DS (2005) Aqueous corrosion of phosphide minerals from iron meteorites: a highly reactive source of prebiotic phosphorus on the surface of the early earth. Astrobiol 5:515–535
Piepenbrink H (1984) Examples of signs of biogenic decomposition in bones long buried. Anthropol Anz 42:241–251
Rabinowitz J, Chang S, Ponnamperuma C (1968) Phosphorylation by way of inorganic phosphate as a potential prebiotic process. Nature 218:442–443
Rye R, Kuo PH, Holland HD (1995) Atmospheric carbon dioxide concentrations before 2.2 billion years ago. Nature 378:603–605
Salimi MH, Heughebaert JC, Nancollas GH (1985) Crystal growth of calcium phosphates in the presence of magnesium ions. Langmuir 1:119–122
Schwartz AW (1971) Phosphate: solubilization and activation on the primitive Earth. In: Buvet R, Ponnamperuma C (eds) Chemical evolution and the origin of life. North Holland, Amsterdam, pp207–215
Schwartz AW, Henderson-Sellers A (1983) Glaciers, volcanic islands and the origin of life. Precambrian Res 22:167–174
Shamsi SA, Danielson ND (1993) Ion chromatography of polyphosphate and polycarboxylates using a napthalenetrisulfonate eluent with indirect photometric and conductivity detection. J Chromatog 653A:153–160
Steinman G, Kenyon DH, Calvin M (1965) Dehydration condensation in aqueous solution. Nature 206:707–708
Tian F, Toon OB, Pavlov AA, De Sterck H (2005) A hydrogen-rich early earth atmosphere. Science 308:1014–1017
Vieyra A, Gueiros-Filho F, Meyer-Fernandes JR, Costa-Sarmento G, de Souza-Barros F (1995) Reactions involving carbamoyl phosphate in the presence of precipitated calcium phosphate with formation of pyrophosphate: a model for primitive energy-conservation pathways. Orig Life Evol Biosph 25:335–350
Walker JCG (1983) Possible limits on the composition of the Archean ocean. Nature 302:518–520
Weber AL (1981) Formation of pyrophosphate, tripolyphosphate and phosphorimidazole with the thioester, N,S-diacetylcysteamine, as the condensing agent. J Mol Evol 18:24–29
Weber AL (1982) Formation of pyrophosphate on hydroxyapatite with thioesters as condensing agents. Biosystems 15:183–189
Yamagata Y (1999) Prebiotic formation of ADP and ATP from AMP, calcium phosphates and cyanate in solution. Orig Life Evol Biosph 29:511–520
Yamagata Y, Mohri T (1982) Formation of cyanate and carbamyl phosphate by electric discharges of model primitive gas. Orig Life Evol Biosph 12:41–44
Acknowledgments
This research was supported by the National Aeronautics and Space Administration through its NSCORT Program at the New York Center for Studies on the Origins of Life.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hagan, W.J., Parker, A., Steuerwald, A. et al. Phosphate Solubility and the Cyanate-Mediated Synthesis of Pyrophosphate. Orig Life Evol Biosph 37, 113–122 (2007). https://doi.org/10.1007/s11084-006-9020-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11084-006-9020-y