Abstract
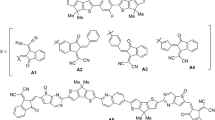
Four Acceptor–Donor-Accepter (A-D-A) type of triangular shaped sub-phthalocyanines (SubPcs) donor molecules namely SubPcs-EDM (sub-phthalocyanines-ethylidene di-malononitrile as M1), SubPcs-ETFM (sub-phthalocyanines-ethylidene tetrafluoro-malononitrile as M2), SubPcs-ETFOM (sub-phthalocyanines-ethylidene tetrafluoro-oxo malononitrile as M3) and SubPcs-EOM (sub-phthalocyanines-ethylidene oxo malononitrile as M4) have been designed for computing its optoelectronic properties with state-of-the-art density functional theory B3LYP/LanL2DZ (d, p) model. In designed molecules, the non-fullerene acceptors are attached at the center of donor moieties. The SubPcs-ETFOM exhibited lowest band gap of energy 2.48 eV with broad absorption band at 645.53 nm. The open circuit voltage (VOC) of M3 is 2.55 V in comparison with phenyl-C60-butyric acid methyl ester which abbreviated as PCBM. This computational study explains that engineered molecules are seemly superb, suitable and suggested for further construction of high performance organic solar cells.
Graphic abstract









Similar content being viewed by others
Data availability
The data which supports the finding of this study will be made available upon reasonable request.
Code availability
This simulation analysis was carried out with Gauss View 5.0 and Gaussian 09 package. The Multiwfn 3.8, VMD 1.9.3, OriginPro 2021, PyMOlyze and PowerPoint 16 has been used for data analyzing and results explanations.
Change history
27 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11082-021-03470-1
References
Abbas, M., Ali, U., Faizan, M., Siddique, M.B.A.: Spirofluorene based small molecules as an alternative to traditional non-fullerene acceptors for organic solar cells. Opt. Quant. Elect. 53(5), 1–14 (2021)
Abbas, F., Ali, U., Muhammad Rizwan Ahmad, H., Tallat, A., Shehzad, A., Zeb, Z., Hussain, I., Saeed, A., Tariq, M.:Role of Iodo-Substituted Subphthalocyanine (Subpcs) π-conjugated aromatic N-fused di-Iminoisonidole units on the performance of non-fullerene small organic solar cells. Comp. Theor. Chem. 1207, 113508 (2022). https://doi.org/10.1016/j.comptc.2021.113508.
Adamo, C., Barone, V.: Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: the m PW and m PW1PW models. J. Chem. Phys. 108(2), 664–675 (1998). https://doi.org/10.1063/1.475428
Afroozeh, A.: Analysis of optical modulator based on silicon waveguide using FDTD. SILICON (2021). https://doi.org/10.1007/s12633-020-00883-7
Ajmal, M., Ali, U., Javed, A., Tariq, A., Arif, Z., Iqbal, J., Shoaib, M., Ahmed, T.: Designing indaceno thiophene–based three new molecules containing non-fullerene acceptors as strong electron withdrawing groups with DFT approaches’. J. Mol. Model. (2019). https://doi.org/10.1007/s00894-019-4198-x
Akbari, E., Buntat, Z., Afroozeh, A., Pourmand, S.E., Farhang, Y., Sanati, P.: Silicene and graphene nano materials in gas sensing mechanism. RSC Adv. 6(85), 81647–81653 (2016). https://doi.org/10.1039/C6RA16736E
Ali, U., Ans, M., Iqbal, J., Iqbal, M.A., Shoaib, M.: Benchmark study of benzamide derivatives and four novel theoretically designed (L1, L2, L3, and L4) ligands and evaluation of their biological properties by DFT approaches. J. Mol. Model 25(8), 1–9 (2019a). https://doi.org/10.1007/s00894-019-4115-3
Ali, U., Javed, A., Shoaib, M., Kashif, M., Raza, A., Cheng, S.-B., Iqbal, J.: Designing difluoro substituted benzene ring based fullerene free acceptors for small Naphthalene Di-Imide based molecules with DFT approaches. Opt. Quantum Electron 51(10), 1–23 (2019b). https://doi.org/10.1007/s11082-019-2047-x
Ali, U., Javed, A., Tallat, A., Iqbal, J., Raza, A.: Molecular designing of four high performance pyrazine based non-fullerene acceptor materials with naphthalene diimide based small organic solar cells. J. Mol. Model 25(2), 1–12 (2019c). https://doi.org/10.1007/s00894-019-3932-8
Ali, U., Javed, A., Ramzan, H., Shoaib, M., Raza, A., Khalil, M.T., Iqbal, J.: Molecular designing of naphthalene diimide based fullerene free small organic solar cell: Acceptors with high photovoltaic performance by density functional theory. Spectrochim. Acta A Mol. Biomol. Spectrosc 228, 117685 (2020). https://doi.org/10.1016/j.saa.2019.117685
Ali, U., Tariq, A., Kiran, A., Abbas, F., Khalil, M.T.: Tuning the absorption and optoelectronic properties of naphthalene diimide based solar cells with non-fullerene acceptors. Chem. Pap. (2021a). https://doi.org/10.1007/s11696-021-01671-2
Ali, U., Ahmad, H.M.R., Faizan, M., et al.: Designing four naphthalene di-imide based small organic solar cells with 5,6-difluoro-3-oxo-2,3-dihydro-indene non-fullerene acceptors. Opt. Quant. Electron 53, 541 (2021b). https://doi.org/10.1007/s11082-021-03209-y
Beaumont, N., Castrucci, J.S., Sullivan, P., Morse, G.E., Paton, A.S., Lu, Z.-H., Jones, T.S.: Acceptor properties of boron subphthalocyanines in fullerene free photovoltaics. J. Phys. Chem. C 118(27), 14813–14823 (2014)
Bin, H., Yang, Y., Zhang, Z.-G., Ye, L., Ghasemi, M., Chen, S., Xue, L.: 9.73% efficiency nonfullerene all organic small molecule solar cells with absorption-complementary donor and acceptor. J. Am. Chem. Soc. y 139(14), 5085–5094 (2017)
Chai, J.-D., Head-Gordon, M.: Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem 10(44), 6615–6620 (2008)
Claessens, C.G., Gonzalez-Rodriguez, D., Rodríguez-Morgade, M.S., Medina, A., Torres, T.: Subphthalocyanines, subporphyrazines, and subporphyrins: singular nonplanar aromatic systems. Chem. Rev. 114(4), 2192–2277 (2014). https://doi.org/10.1021/cr400088w
Cnops, K., Rand, B.P., Cheyns, D., Verreet, B., Empl, M.A., Heremans, P.: 8.4% efficient fullerene-free organic solar cells exploiting long-range exciton energy transfer. Nat. Commun. 5(1), 1–6 (2014)
Duan, C., Zango, G., García Iglesias, M., Colberts, F.J., Wienk, M.M., Martínez-Díaz, M.V., Torres, T.: The role of the axial substituent in subphthalocyanine acceptors for bulk-heterojunction solar cells. Angew. Chem. 129(1), 154–158 (2017). https://doi.org/10.1002/ange.201608644
Ebenhoch, B., Prasetya, N.B., Rotello, V.M., Cooke, G., Samuel, I.D.: Solution-processed boron subphthalocyanine derivatives as acceptors for organic bulk-heterojunction solar cells. J. Mater. Chem. 3(14), 7345–7352 (2015). https://doi.org/10.1039/C5TA00715A
Günes, S., Neugebauer, H., Sariciftci, N.S.: Conjugated polymer-based organic solar cells. Chem. Rev. 107(4), 1324–1338 (2007). https://doi.org/10.1021/cr050149z
Hertwig, R.H., Koch, W.: On the parameterization of the local correlation functional What is Becke-3-LYP? Chem. Phys. Lett. (1997). https://doi.org/10.1016/S0009-2614(97)00207-8
Holliday, S., Ashraf, R.S., Wadsworth, A., Baran, D., Yousaf, S.A., Nielsen, C.B., Gasparini, N.: High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 7(1), 1–11 (2016). https://doi.org/10.1038/ncomms11585|www.nature.com/naturecommunications
Hussain, Z., Shoaib, M., Ali, U., Ramzan, H., Ali, M., Tariq, A., Naqash, M.: Theoretical study of isoxazoles and their derivatives for evaluating its pharmaceutical properties with density functional theory’. J Comput Chem Mol Model (2020). https://doi.org/10.25177/JCCMM.4.3.RA.10623
Iftikhar, T., Ali, U., Shoaib, M.: Theoretical study of α, β unsaturated carbonyl thiophene derivatives to investigate optoelectronic properties toward organic photovoltaics. J. Mol. Model. 26(12), 1–8 (2020)
Kiran, A., Ali, U., Hussain, A., et al.: Molecular designing of tetra-aryl-p-benzoquinones derivatives toward strong optical properties. Chem. Pap. 75, 6661–6671 (2021). https://doi.org/10.1007/s11696-021-01834-1
Li, G., Zhu, R., Yang, Y.: Polymer solar cells. Nat. Photonics 6(3), 153–161 (2012). https://doi.org/10.1038/nphoton.2012.11
Rassolov, V.A., Ratner, M.A., Pople, J.A., Redfern, P.C., Curtiss, L.A.: 6–31G* basis set for third-row atoms. J. Comput. Chem. 22(9), 976–984 (2001). https://doi.org/10.1002/jcc.1058
Ripaud, E., Rousseau, T., Leriche, P., Roncali, J.: Unsymmetrical Triphenylamine-Oligothiophene Hybrid Conjugated Systems as Donor Materials for High-Voltage Solution-Processed Organic Solar Cells. Adv. Energy Mater. 1(4), 540–545 (2011). https://doi.org/10.1002/aenm.201100065
Yanai, T., Tew, D.P., Handy, N.C.: A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 393(1–3), 51–57 (2004). https://doi.org/10.1021/cr9904009
Zhang, J., Zhang, Y., Fang, J., Lu, K., Wang, Z., Ma, W., Wei, Z.: Conjugated polymer–small molecule alloy leads to high efficient ternary organic solar cells. J. Am. Chem. Soc. 137(25), 8176–8183 (2015). https://doi.org/10.1021/jacs.5b03449
Acknowledgements
The authors acknowledge the technical supports provided by Department of Chemistry, University of Agriculture Faisalabad, Faisalabad, 38040, Pakistan and Punjab Bioenergy Institute, Jhang Road, Faisalabad, 38040, Pakistan.
Author information
Authors and Affiliations
Contributions
The authors Faheem Abbas and Usman Ali equally contributed for this research study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original publication of the article, the authors noticed errors in abstract, figure caption, reference, layout of tables and figures. These errors have been corrected now.
Rights and permissions
About this article
Cite this article
Abbas, F., Ali, U., Ahmad, H.M.R. et al. Body centered non-fullerene acceptors substitution on triangular shaped Sub-phthalocyanines (SubPcs) based A-D-A organic solar cells: A step toward new strategies for better performances. Opt Quant Electron 54, 21 (2022). https://doi.org/10.1007/s11082-021-03413-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-021-03413-w