Abstract
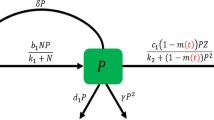
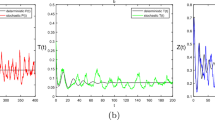
The increasing input of environmental toxins in aquatic systems raises concerns regarding the environmental exposure and impact of toxins on natural aquatic environments. Phytoplankton and zooplankton appear to be among the most sensitive aquatic organisms to environmental toxins. Moreover, toxin-producing phytoplankton plays an important role in regulating the real aquatic ecosystems. In this paper, the combined effects of these factors on the dynamics of phytoplankton–zooplankton interactions are investigated. The phytoplankton grows logistically, but their growth rate is suppressed due to the presence of environmental toxins. The zooplankton is assumed to be generalist and follows logistic growth in the absence of phytoplankton. Also, it is considered that toxicants in the environment are increased constantly due to different natural and human behaviors. Global sensitivity analysis helps to identify the most significant parameters that reduce the environmental toxins in the system. Among these, the input rate of environmental toxins, contact rate between phytoplankton and environmental toxins, and environmental toxins-induced growth suppression of phytoplankton have destabilizing effect on the dynamics of system, while the depletion rate of environmental toxins has stabilizing effect. Therefore, it is imperative to modulate the depletion rate of environmental toxins to prevent the crash of the aquatic food web system. Further, we incorporate seasonal variations in the model, letting the parameters become functions of time. Sufficient conditions for the existence and stability of positive periodic solutions are obtained. We also derive conditions for existence, uniqueness and stability of a positive almost periodic solution. Large values of time-dependent toxin release by phytoplankton and input rate of environmental toxins induce periodic solutions of the nonautonomous system while the corresponding autonomous system exhibits a stable focus. Interestingly, our nonautonomous system exhibits bursting patterns for two slow rationally related excitation frequencies. Finally, we convert our deterministic autonomous model into stochastic model by introducing additive noise term. We find that the stability of the system gets disturbed in the presence of environmental fluctuation.













Similar content being viewed by others
References
Riley, R.A., Stommel, H., Burrpus, D.P.: Qualitative ecology of the plankton of the Western North Atlantic. Bull. Bingham Oceanogr. Collect. Yale Univ. 12, 1–169 (1949)
Chen, M., et al.: The dynamics of temperature and light on the growth of phytoplankton. J. Theor. Biol. 385, 8–19 (2015)
Sekerci, Y., Petrovskii, S.: Mathematical modelling of plankton–oxygen dynamics under the climate change. Bull. Math. Biol. 77, 2325–2353 (2015)
Moss, B.R.: Ecology of fresh waters: man and medium, past to future, 3rd edn. Wiley-Blackwell (2009)
Edwards, A.M., Brindley, J.: Oscillatory behavior in a three component plankton population model. Dyn. Stab. Syst. 11, 347–370 (1996)
Behrenfeld, M.J., Falkowski, P.G.: A consumer’s guide to phytoplankton primary productivity models. Limnol. Oceanogr. 42, 1479–1491 (1997)
Hoppe, G., et al.: Bacterial growth and primary production along a north-south transect of the Atlantic Ocean. Nature 416, 168–171 (2002)
Huppert, A., et al.: A model for seasonal phytoplankton blooms. J. Theor. Biol. 236, 276–290 (2005)
Yang, X., et al.: Stability and bifurcation in a stoichiometric producer-grazer model with knife edge. SIAM J. Appl. Dyn. Syst. 15, 2051–2077 (2016)
Chakraborty, S., Roy, S., Chattopadhyay, J.: Nutrient-limited toxin production and the dynamics of two phytoplankton in culture media: a mathematical model. Ecol. Model. 213, 191–201 (2008)
Das, K., Ray, S.: Effect of delay on nutrient cycling in phytoplankton–zooplankton interactions in estuarine system. Ecol. Model. 215, 69–76 (2008)
Jang, S.J., Baglama, J., Rick, J.: Nutrient–phytoplankton–zooplankton models with a toxin. Math. Comput. Model. 43(1/2), 105–118 (2006)
Panja, P., Mondal, S.K.: Stability analysis of coexistence of three species prey–predator model. Nonlinear Dyn. 81(1–2), 373–382 (2015)
Mukhopadhyay, B., Bhattacharyya, R.: Modelling phytoplankton allelopathy in a nutrient–plankton model with spatial heterogeneity. Ecol. Model. 198, 163–173 (2006)
Roy, S.: The coevolution of two phytoplankton species on a single resource: allelopathy as a pseudo-mixotrophy. Theor. Popul. Biol. 75, 68–75 (2009)
Silva, M.D., Jang, S.R.-J.: Dynamical behavior of systems of two phytoplankton and one zooplankton populations with toxin producing phytoplankton. Math. Methods Appl. Sci. 40, 4295–4309 (2017)
Cronberg, G.: Changes in the phytoplankton of Lake Trummen included by restoration. Hydrobiologia 86, 185–193 (1982)
Leah, R.T., Moss, B., Forrest, D.E.: The role of predation in causing major changes in the limnology. Int. Revue ges. Hydrobiol. 65(2), 223–247 (1990)
Scheffer, M.: Alternative stable states in eutrophic shallow fresh water systems: a minimal model. Hydrobiol. Bull. 23, 73–85 (1989)
Moss, B.: Engineering and biological approaches to the restoration from eutrophication of shallow lakes in which aquatic plant communities are important components. Hydrobiol. 200(201), 367–377 (1990)
Boney, A.D.: Phytoplankton. Edward Arnold Ltd., London (1976)
Odum, E.P.: Fundamentals of Ecology. W. B. Saunders Company, Philadelphia (1971)
Kirk, K., Gilbert, J.: Variations in herbivore response to chemical defences: zooplankton foraging on toxic cyanobacteria. Ecology 73, 2208–2213 (1992)
Kozlowsky-Suzuki, B., et al.: Feeding, reproduction and toxin accumulation by the copepods Acartia bifilosa and Eurytemora affinis in the presence of the toxic cyanobacterium Nodularia spumigena. Mar. Ecol. Prog. Ser. 249, 237–249 (2003)
Liu, C., et al.: Dynamic analysis of a hybrid bioeconomic plankton system with double time delays and stochastic fluctuations. Appl. Math. Comput. 316, 115–137 (2018)
Chattopadhyay, J.: Effect of toxic substances on a two-species competitive system. Ecol. Model. 84, 287–289 (1996)
Chattopadhayay, J., Sarkar, R.R., Mandal, S.: Toxin producing plankton may act as a biological control for planktonic blooms-field study and mathematical modeling. J. Theor. Biol. 215(3), 333–344 (2002)
Sarkar, R.R., Chattopadhayay, J.: Occurrence of planktonic blooms under environmental fluctuations and its possible control mechanism—mathematical models and experimental observations. J. Theor. Biol. 224, 501–516 (2003)
Pal, R., Basu, D., Banerjee, M.: Modelling of phytoplankton allelopathy with Monod–Haldane-type functional response—a mathematical study. Biosystems 95, 243–253 (2009)
Schmidt, L.E., Hansen, P.J.: Allelopathy in the prymnesiophyte Chyrsochromulina polylepis: effect of cell concentration, growth phase and pH. Mar. Ecol. Prog. Ser. 216, 67–81 (2001)
Windust, A.J., Wright, J.L.C., McLachlan, J.L.: The effects of the diarrhetic shellfish poisoning toxins okadaic acid and dinophysistoxin-1, on the growth of microalgae. Mar. Biol. 126, 19–25 (1996)
Hansen, F.C.: Trophic interaction between zooplankton and Phaeocystis cf. globosa. Helgol. Meeresunters. 49, 283–293 (1995)
Nielsen, T.G., Kiorboe, T., Bjornsen, P.K.: Effects of a Chrysochromulina polylepis sub surface bloom on the plankton community. Mar. Ecol. Prog. Ser. 62, 21–35 (1990)
Buskey, E.J., Hyatt, C.J.: Effect of the Texas (USA) brown tide alga on planktonic grazers. Mar. Ecol. Prog. Ser. 126, 285–292 (1995)
Banerjee, M., Venturino, E.: A phytoplankton–toxic phytoplankton–zooplankton model. Ecol. Complex. 8, 239–248 (2011)
Moratou-Apostolopoulou, M., Ignatiades, L.: Pollution effects on the phytoplankton–zooplankton relationships in an inshore environment. Hydrobiologia 75(2), 259–266 (1980)
Tchounwou, P.B., et al.: Heavy metals toxicity and the environment. Exs 101, 133–164 (2012)
Huang, Y.J., et al.: The chronic effects of oil pollution on marine phytoplankton in a subtropical bay. Chin. Environ. Monit. Assess. 176(1), 517–530 (2011)
U.S. Environmental Protection Agency Great Lkes National Program Office Significant Activities Report. http://www.epa.gov/glnpo/aoc/waukegan.html
Labille, J., Brant, J.: Stability of nanoparticles in water. Nanomedicine 5(6), 985–998 (2010)
Miao, A.J., et al.: The algal toxicity of silver engineered nanoparticles and detoxification by exopolymeric substances. Environ. Pollut. 157, 3034–3041 (2009)
Miao, A., et al.: Zinc oxide-engineered nanoparticles: dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 29(12), 2814–2822 (2010)
Miller, R.J., et al.: \(\text{ TiO }_2\) nanoparticles are phototoxic to marine phytoplankton. PLoS ONE 7(1), e30321 (2012)
Rana, S., et al.: The effect of nanoparticles on plankton dynamics: a mathematical model. BioSystems 127, 28–41 (2015)
Panja, P., Mondal, S.K., Jana, D.K.: Effects of toxicants on Phytoplankton–Zooplankton–Fish dynamics and harvesting. Chaos Solit. Fract. 104, 389–399 (2017)
Chakraborty, S., Chattopadhyay, J.: Nutrient–phytoplankton–zooplankton dynamics in the presence of additional food source—a mathematical study. J. Biol. Syst. 16(04), 547–564 (2008)
Roy, S., Chattopadhyay, J.: Disease-selective predation may lead to prey extinction. Math. Methods Appl. Sci. 28, 1257–1267 (2005)
Das, K.P., Roy, S., Chattopadhyay, J.: Effect of disease-selective predation on prey infected by contact and external sources. BioSystems 95, 188–199 (2009)
Timms, R.M., Moss, B.: Prevention of growth of potentially dense phytoplankton populations by zooplankton grazing, in the presence of zooplanktivorous fish, in a shallow wetland ecosystem. Limnol. Oceanogr. 29, 472–486 (1984)
Murdoch, W.W., Bence, J.: General predators and unstable prey populations -. In: Kerfoot, W.C., Sih, A. (eds.) Predation: Direct and Indrect Impacts on Aquatic Communities, pp. 17–30. University Press of New England, Hanover (1987)
Yu, X., Yuan, S., Zhang, T.: Survival and ergodicity of a stochastic phytoplankton–zooplankton model with toxin-producing phytoplankton in an impulsive polluted environment. Appl. Math. Comput. 347, 249–264 (2019)
Jang, S.R.-J., Baglama, J., Rick, J.: Plankton–toxin interaction with a variable input nutrient. J. Biol. Dyn. 2(1), 14–30 (2008)
Chakraborty, S., Chatterjee, S., Venturino, E., Chattopadhyay, J.: Recurring plankton bloom dynamics modeled via toxin-Producing phytoplankton. J. Biol. Phys. 33(4), 271–290 (2007)
Rueter, J.R., Chisholm, S.W., Morel, F.: Effects of copper toxicity on silicon acid uptake and growth in Thalassiosira pseudonana. J. Phycol. 17, 270–278 (1981)
Vardi, A., et al.: Dinoflagellate-cyanobacterium communication may determine the composition of phytoplankton assemblage in a mesotrophic lake. Curr. Biol. 12(20), 1767–1772 (2002)
Scheffer, M.: Fish and nutrients interplay determines algal biomass: a minimal model. Oikos 62, 271–282 (1991)
Beretta, E., Kuang, Y.: Modelling and analysis of a marine bacteriophase infection. Math. Biosci. 149, 57–67 (1998)
Abate, A., Tiwari, A., Sastry, S.: Box invariance in biologically-inspired dynamical systems. Automatica 45, 1601–1610 (2009)
Lakshmikantham, V., Leela, S., Martynyuk, A.A.: Stability Analysis of Nonlinear Systems. Marcel Dekker, Inc., New York (1989)
Birkhoff, G., Rota, G.C.: Ordinary Differential Equations, 4th edn. Wiley, New York (1989)
Hassard, B., Kazarinoff, N., Wan, Y.: Theory and Application of Hopf-Bifurcation. London Mathematical Society Lecture Note Series. Cambridge University Press, Cambridge (1981)
Graneli, E., Johansson, N.: Effects of the toxic haptophyte Prymnesium parvum on the survival and feeding of a ciliate: the influence of different nutrient conditions. Mar. Ecol. Prog. Ser. 254, 49–56 (2003)
Johansson, N., Graneli, E.: Influence of different nutrient conditions on cell density, chemical composition and toxicity of Prymnesium parvum (Haptophyta) in semi-continuous cultures. J. Exp. Mar. Biol. Ecol. 239, 243–258 (1999)
Gopalsamy, K.: Stability and Oscillations in Delay Differential Equations of Population Dynamics. Kluwer Academic Publishers, Boston (1992)
Yoshizawa, T.: Stability Theory and the Existence of Periodic Solutions and Almost Periodic Solutions, vol. 14. Springer, New York (2012)
Blower, S.M., Dowlatabadi, M.: Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int. Stat. Rev. 62, 229–243 (1994)
Marino, S., et al.: A methodology for performing global uncertainty and sensitivity analysis in systems biology. J. Theor. Biol. 254(1), 178–196 (2008)
Izhikevich, E.M., et al.: Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci. 26, 161–167 (2003)
Schuster, S., Knoke, B., Marhl, M.: Differential regulation of proteins by bursting calcium oscillations—a theoretical study. BioSystems 81, 49–63 (2005)
Li, X.H., Bi, Q.S.: Single-Hopf bursting in periodic perturbed Belousov—Zhabotinsky reaction with two time scales. Chin. Phys. Lett. 30, 010503–6 (2013)
Kingni, S.T., et al.: Bursting oscillations in a 3d system with asymmetrically distributed equilibria: mechanism, electronic implementation and fractional derivation effect. Chaos Solit. Fract. 71, 29–40 (2015)
Ibarz, B., Casado, J.M., Sanjun, M.A.F.: Map-based models in neuronal dynamics. Phys. Rep. 501, 1–74 (2011)
Rinzel, J.: Bursting oscillation in an excitable membrane model. In: Sleeman, B.D., Jarvis, R.J. (eds.) Ordinary and Partial Differential Equations, pp. 304–316. Springer, Berlin (1985)
Izhikevich, E.M.: Neural excitability, spiking and bursting. Int. J. Bifurc. Chaos 10(06), 1171–1266 (2000)
Han, X., et al.: Frequency-truncation fast-slow analysis for parametrically and externally excited systems with two slow incommensurate excitation frequencies. Commun. Nonlinear Sci. Numer. Simulat. 72, 16–25 (2019)
Wang, C., Tang, J., Ma, J.: Minireview on signal exchange between nonlinear circuits and neurons via field coupling. Eur. Phys. J. Spec. Top. 228, 1907–1924 (2019)
Han, X., et al.: Origin of mixed-mode oscillations through speed escape of attractors in a Rayleigh equation with multiple-frequency excitations. Nonlinear Dyn. 88, 2693–2703 (2017)
Han, X., et al.: Fast-slow analysis for parametrically and externally excited systems with two slow rationally related excitation frequencies. Phy. Rev. E 92, 012911 (2015)
Han, X., Bi, Q., Kurths, J.: Route to bursting via pulse-shaped explosion. Phy. Rev. E 98, 010201(R) (2018)
Yu, X., Yuan, S., Zhang, T.: The effects of toxin-producing phytoplankton and environmental fluctuations on the planktonic blooms. Nonlinear Dyn. 91(3), 1653–1668 (2018)
Lan, G., Wei, C., Zhang, S.: Long time behaviors of single-species population models with psychological effect and impulsive toxicant in polluted environments. Phys. A 521, 528–542 (2019)
Higham, D.J.: An algorithmic introduction to numerical simulation of stochastic differential equations. SIAM 43, 525–546 (2001)
Navarro, E., et al.: Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 42(23), 8959–8964 (2008)
Chhater, A., et al.: Bacterial consortia for crude oil spill remediation. Water Sci. Technol. 34, 187–193 (1996)
Duval, B.D.: UV from Sunlight Excites Nanoparticles to Kill Phytoplankton in Lab Setting (2012). http://earthsky.org/human-world/uv-from-sunlight-excites-nanoparticles-to-kill-phytoplankton-in-lab-setting/
Acknowledgements
The authors express their gratitude to the associate editor and the reviewers whose comments and suggestions have helped the improvements of this paper.
Funding
The research work of Arindam Mandal is supported by University Grants Commission in the form of Senior Research Fellowship (Ref. No: 19/06/2016(i)EU-V). Pankaj Kumar Tiwari is thankful to University Grants Commissions, New Delhi, India, for providing financial support in form of D.S. Kothari postdoctoral fellowship (No. F.4-2/2006 (BSR)/MA/17-18/0021). Research of Samares Pal is partially supported by DST-FIST programme of University of Kalyani (474 (Sanc.)/ST/P/S & T/16 G-22/2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Ethical standard
The authors state that this research complies with ethical standards. This research does not involve either human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mandal, A., Tiwari, P.K., Samanta, S. et al. A nonautonomous model for the effect of environmental toxins on plankton dynamics. Nonlinear Dyn 99, 3373–3405 (2020). https://doi.org/10.1007/s11071-020-05480-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-020-05480-2