Abstract
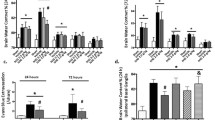
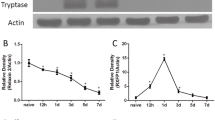
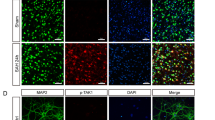
The degranulation of mast cells accounts for the development of neuroinflammation following intracerebral hemorrhage (ICH). Inhibition of IRE1α, a sensor signaling protein related to endoplasmic reticulum stress, has been shown to exert anti-inflammatory effects in several neurological diseases. The objective of this study was to investigate the effects of IRE1α inhibition on mast cells degranulation in an ICH mouse model and to explore the contribution of miR-125/Lyn pathway in IRE1α-mediated mast cells degranulation. Male mice were subjected to ICH by intraparenchymal injection of autologous blood. STF083010, an inhibitor of IRE1α, was administered intranasally at 1 h after ICH induction. AntimiR-125 was delivered by intracerebroventricular (i.c.v.) injection prior to ICH induction to elucidate the possible mechanisms. Western blot analysis, immunofluorescence staining, neurological test, hematoma volume, brain water content, toluidine blue staining and reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) were performed. Endogenous phosphorylated IRE1α (p-IRE1α), tryptase, interleukin-17A (IL-17A), tumor necrosis factor α (TNF-α) and tryptase mRNA were increased in time dependent manner while miR-125b-2-3p was decreased after ICH. Inhibition of IRE1α, with STF083010, remarkably reduced brain water content, improved neurological function, decreased hematoma volume, upregulated the expression of miR-125b-2-3p, decreased the number of mast cells, and downregulated the protein expression of Lyn kinase, XBP1s (spliced X-box binding protein-1), tryptase, IL-17A and TNF-α. The downregulation of Lyn kinase, tryptase, IL-17A, TNF-α, and decreased mast cells number were reversed by antimiR-125. The present findings demonstrate that IRE1α inhibition attenuates mast cells degranulation and neuroinflammation, at least partially, through IRE1α/miR-125/Lyn signaling pathway after ICH.








Similar content being viewed by others
Data Availability
Enquiries about data availability should be directed to the authors.
References
Hemphill JC 3rd, Adeoye OM, Alexander DN, Alexandrov AW, Amin-Hanjani S, Cushman M, George MG, LeRoux PD, Mayer SA, Qureshi AI, Saver JL, Schwamm LH, Sheth KN, Tirschwell D (2018) Clinical performance measures for adults hospitalized with intracerebral hemorrhage: performance measures for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 49(7):e243–e261. https://doi.org/10.1161/str.0000000000000171
Ren H, Han R, Chen X, Liu X, Wan J, Wang L, Yang X, Wang J (2020) Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: an update. J Cereb Blood Flow Metab 40(9):1752–1768. https://doi.org/10.1177/0271678x20923551
Manaenko A, Lekic T, Ma Q, Zhang JH, Tang J (2013) Hydrogen inhalation ameliorated mast cell-mediated brain injury after intracerebral hemorrhage in mice. Crit Care Med 41(5):1266–1275. https://doi.org/10.1097/CCM.0b013e31827711c9
Parrella E, Porrini V, Benarese M, Pizzi M (2019) The role of mast cells in stroke. Cells 8(5):437. https://doi.org/10.3390/cells8050437
Yehya M, Torbey MT (2018) The role of mast cells in intracerebral hemorrhage. Neurocrit Care 28(3):288–295. https://doi.org/10.1007/s12028-017-0416-5
Nelissen S, Lemmens E, Geurts N, Kramer P, Maurer M, Hendriks J, Hendrix S (2013) The role of mast cells in neuroinflammation. Acta Neuropathol 125(5):637–650. https://doi.org/10.1007/s00401-013-1092-y
Theoharides TC, Tsilioni I, Patel AB, Doyle R (2016) Atopic diseases and inflammation of the brain in the pathogenesis of autism spectrum disorders. Transl Psychiatry 6(6):e844. https://doi.org/10.1038/tp.2016.77
Skaper SD, Facci L, Giusti P (2014) Neuroinflammation, microglia and mast cells in the pathophysiology of neurocognitive disorders: a review. CNS Neurol Disord Drug Targets 13(10):1654–1666. https://doi.org/10.2174/1871527313666141130224206
Ocak U, Eser Ocak P, Huang L, Xu W, Zuo Y, Li P, Gamdzyk M, Zuo G, Mo J, Zhang G, Zhang JH (2020) Inhibition of mast cell tryptase attenuates neuroinflammation via PAR-2/p38/NFκB pathway following asphyxial cardiac arrest in rats. J Neuroinflammation 17(1):144. https://doi.org/10.1186/s12974-020-01808-2
Dahlin JS, Hallgren J (2015) Mast cell progenitors: origin, development and migration to tissues. Mol Immunol 63(1):9–17. https://doi.org/10.1016/j.molimm.2014.01.018
Silver R, Curley JP (2013) Mast cells on the mind: new insights and opportunities. Trends Neurosci 36(9):513–521. https://doi.org/10.1016/j.tins.2013.06.001
Rodewald HR, Dessing M, Dvorak AM, Galli SJ (1996) Identification of a committed precursor for the mast cell lineage. Science 271(5250):818–822. https://doi.org/10.1126/science.271.5250.818
Gilfillan AM, Beaven MA (2011) Regulation of mast cell responses in health and disease. Crit Rev Immunol 31(6):475–529. https://doi.org/10.1615/critrevimmunol.v31.i6.30
Gilfillan AM, Austin SJ, Metcalfe DD (2011) Mast cell biology: introduction and overview. Adv Exp Med Biol 716:2–12. https://doi.org/10.1007/978-1-4419-9533-9_1
Rivera J, Fierro NA, Olivera A, Suzuki R (2008) New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol 98:85–120. https://doi.org/10.1016/s0065-2776(08)00403-3
Furumoto Y, Gonzalez-Espinosa C, Gomez G, Kovarova M, Odom S, Parravicini V, Ryana JJ, Rivera J (2004) Rethinking the role of Src family protein tyrosine kinases in the allergic response: new insights on the functional coupling of the high affinity IgE receptor. Immunol Res 30(2):241–253. https://doi.org/10.1385/ir:30:2:241
Mohammed Thangameeran SI, Tsai ST, Hung HY, Hu WF, Pang CY, Chen SY, Liew HK (2020) A role for endoplasmic reticulum stress in intracerebral hemorrhage. Cells 9(3):750. https://doi.org/10.3390/cells9030750
Duan XC, Wang W, Feng DX, Yin J, Zuo G, Chen DD, Chen ZQ, Li HY, Wang Z, Chen G (2017) Roles of autophagy and endoplasmic reticulum stress in intracerebral hemorrhage-induced secondary brain injury in rats. CNS Neurosci Ther 23(7):554–566. https://doi.org/10.1111/cns.12703
Senft D, Ronai ZA (2015) UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci 40(3):141–148. https://doi.org/10.1016/j.tibs.2015.01.002
Burman A, Tanjore H, Blackwell TS (2018) Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol 68–69:355–365. https://doi.org/10.1016/j.matbio.2018.03.015
He Y, Sun S, Sha H, Liu Z, Yang L, Xue Z, Chen H, Qi L (2010) Emerging roles for XBP1, a sUPeR transcription factor. Gene Expr 15(1):13–25. https://doi.org/10.3727/105221610x12819686555051
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334(6059):1081–1086. https://doi.org/10.1126/science.1209038
Morris G, Puri BK, Walder K, Berk M, Stubbs B, Maes M, Carvalho AF (2018) The endoplasmic reticulum stress response in neuroprogressive diseases: emerging pathophysiological role and translational implications. Mol Neurobiol 55(12):8765–8787. https://doi.org/10.1007/s12035-018-1028-6
Zhao W, Han F, Shi Y (2016) IRE1α pathway of endoplasmic reticulum stress induces neuronal apoptosis in the locus coeruleus of rats under single prolonged stress. Prog Neuropsychopharmacol Biol Psychiatry 69:11–18. https://doi.org/10.1016/j.pnpbp.2016.03.008
Ghosh R, Wang L, Wang ES, Perera BG, Igbaria A, Morita S, Prado K, Thamsen M, Caswell D, Macias H, Weiberth KF, Gliedt MJ, Alavi MV, Hari SB, Mitra AK, Bhhatarai B, Schürer SC, Snapp EL, Gould DB, German MS, Backes BJ, Maly DJ, Oakes SA, Papa FR (2014) Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell 158(3):534–548. https://doi.org/10.1016/j.cell.2014.07.002
Feldman HC, Tong M, Wang L, Meza-Acevedo R, Gobillot TA, Lebedev I, Gliedt MJ, Hari SB, Mitra AK, Backes BJ, Papa FR, Seeliger MA, Maly DJ (2016) Structural and functional analysis of the allosteric inhibition of IRE1α with ATP-competitive ligands. ACS Chem Biol 11(8):2195–2205. https://doi.org/10.1021/acschembio.5b00940
Li X, Han F, Shi Y (2015) IRE1α-XBP1 pathway is activated upon induction of single-prolonged stress in rat neurons of the medial prefrontal cortex. J Mol Neurosci 57(1):63–72. https://doi.org/10.1007/s12031-015-0577-7
van Kralingen JC, McFall A, Ord ENJ, Coyle TF, Bissett M, McClure JD, McCabe C, Macrae IM, Dawson J, Work LM (2019) Altered extracellular vesicle microRNA expression in ischemic stroke and small vessel disease. Transl Stroke Res 10(5):495–508. https://doi.org/10.1007/s12975-018-0682-3
Moon GJ, Sung JH, Kim DH, Kim EH, Cho YH, Son JP, Cha JM, Bang OY (2019) Application of mesenchymal stem cell-derived extracellular vesicles for stroke: biodistribution and microRNA study. Transl Stroke Res 10(5):509–521. https://doi.org/10.1007/s12975-018-0668-1
Burek M, König A, Lang M, Fiedler J, Oerter S, Roewer N, Bohnert M, Thal SC, Blecharz-Lang KG, Woitzik J, Thum T, Förster CY (2019) Hypoxia-induced microRNA-212/132 alter blood-brain barrier integrity through inhibition of tight junction-associated proteins in human and mouse brain microvascular endothelial cells. Transl Stroke Res 10(6):672–683. https://doi.org/10.1007/s12975-018-0683-2
Tapeh BEG, Alivand MR, Solali S (2020) The role of microRNAs in acute lymphoblastic leukaemia: from biology to applications. Cell Biochem Funct 38(4):334–346. https://doi.org/10.1002/cbf.3466
Omidkhoda N, Wallace Hayes A, Reiter RJ, Karimi G (2019) The role of MicroRNAs on endoplasmic reticulum stress in myocardial ischemia and cardiac hypertrophy. Pharmacol Res 150:104516. https://doi.org/10.1016/j.phrs.2019.104516
Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, Kellner CP, Hahn DK, Merkow MB, Garrett MC, Starke RM, Cho BM, Sosunov SA, Connolly ES (2008) A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc 3(1):122–128. https://doi.org/10.1038/nprot.2007.513
Zhang C, Jiang M, Wang WQ, Zhao SJ, Yin YX, Mi QJ, Yang MF, Song YQ, Sun BL, Zhang ZY (2020) Selective mGluR1 negative allosteric modulator reduces blood-brain barrier permeability and cerebral edema after experimental subarachnoid hemorrhage. Transl Stroke Res 11(4):799–811. https://doi.org/10.1007/s12975-019-00758-z
Huang J, Liu W, Doycheva DM, Gamdzyk M, Lu W, Tang J, Zhang JH (2019) Ghrelin attenuates oxidative stress and neuronal apoptosis via GHSR-1α/AMPK/Sirt1/PGC-1α/UCP2 pathway in a rat model of neonatal HIE. Free Radic Biol Med 141:322–337. https://doi.org/10.1016/j.freeradbiomed.2019.07.001
Zhu Q, Enkhjargal B, Huang L, Zhang T, Sun C, Xie Z, Wu P, Mo J, Tang J, Xie Z, Zhang JH (2018) Aggf1 attenuates neuroinflammation and BBB disruption via PI3K/Akt/NF-κB pathway after subarachnoid hemorrhage in rats. J Neuroinflammation 15(1):178. https://doi.org/10.1186/s12974-018-1211-8
Xie Z, Huang L, Enkhjargal B, Reis C, Wan W, Tang J, Cheng Y, Zhang JH (2017) Intranasal administration of recombinant Netrin-1 attenuates neuronal apoptosis by activating DCC/APPL-1/AKT signaling pathway after subarachnoid hemorrhage in rats. Neuropharmacology 119:123–133. https://doi.org/10.1016/j.neuropharm.2017.03.025
Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Raikwar SP, Zaheer SA, Iyer SS, Burton C, James D, Zaheer A (2020) Mast cell activation, neuroinflammation, and tight junction protein derangement in acute traumatic brain injury. Mediators Inflamm 2020:4243953. https://doi.org/10.1155/2020/4243953
Norton WT, Aquino DA, Hozumi I, Chiu FC, Brosnan CF (1992) Quantitative aspects of reactive gliosis: a review. Neurochem Res 17(9):877–885. https://doi.org/10.1007/bf00993263
Ziemka-Nalecz M, Jaworska J, Zalewska T (2017) Insights into the neuroinflammatory responses after neonatal hypoxia-ischemia. J Neuropathol Exp Neurol 76(8):644–654. https://doi.org/10.1093/jnen/nlx046
Li P, Zhao G, Chen F, Ding Y, Wang T, Liu S, Lu W, Xu W, Flores J, Ocak U, Zhang T, Zhang JH, Tang J (2020) Rh-relaxin-2 attenuates degranulation of mast cells by inhibiting NF-κB through PI3K-AKT/TNFAIP3 pathway in an experimental germinal matrix hemorrhage rat model. J Neuroinflamm 17(1):250. https://doi.org/10.1186/s12974-020-01926-x
Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML (2010) Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab 30(4):689–702. https://doi.org/10.1038/jcbfm.2009.282
Akyol GY, Manaenko A, Akyol O, Solaroglu I, Ho WM, Ding Y, Flores J, Zhang JH, Tang J (2017) IVIG activates FcγRIIB-SHIP1-PIP3 Pathway to stabilize mast cells and suppress inflammation after ICH in mice. Sci Rep 7(1):15583. https://doi.org/10.1038/s41598-017-15455-w
Strbian D, Tatlisumak T, Ramadan UA, Lindsberg PJ (2007) Mast cell blocking reduces brain edema and hematoma volume and improves outcome after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab 27(4):795–802. https://doi.org/10.1038/sj.jcbfm.9600387
Wang JK, Wang Z, Li G (2019) MicroRNA-125 in immunity and cancer. Cancer Lett 454:134–145. https://doi.org/10.1016/j.canlet.2019.04.015
Lee HM, Kim TS, Jo EK (2016) MiR-146 and miR-125 in the regulation of innate immunity and inflammation. BMB Rep 49(6):311–318. https://doi.org/10.5483/bmbrep.2016.49.6.056
Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, Papa FR, Oakes SA (2012) IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science 338(6108):818–822. https://doi.org/10.1126/science.1226191
Huang J, Lu W, Doycheva DM, Gamdzyk M, Hu X, Liu R, Zhang JH, Tang J (2020) IRE1α inhibition attenuates neuronal pyroptosis via miR-125/NLRP1 pathway in a neonatal hypoxic-ischemic encephalopathy rat model. J Neuroinflamm 17(1):152. https://doi.org/10.1186/s12974-020-01796-3
Su R, Zhao E, Zhang J (2021) miR-496 inhibits proliferation via LYN and AKT pathway in gastric cancer. Open Med (Wars) 16(1):1206–1214. https://doi.org/10.1515/med-2021-0313
Liu Y, Dong J, Mu R, Gao Y, Tan X, Li Y, Li Z, Yang G (2013) MicroRNA-30a promotes B cell hyperactivity in patients with systemic lupus erythematosus by direct interaction with Lyn. Arthritis Rheum 65(6):1603–1611. https://doi.org/10.1002/art.37912
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC 81601051, NSFC 82171457).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, Z., Huang, J., Liao, Y. et al. ER Stress is Involved in Mast Cells Degranulation via IRE1α/miR-125/Lyn Pathway in an Experimental Intracerebral Hemorrhage Mouse Model. Neurochem Res 47, 1598–1609 (2022). https://doi.org/10.1007/s11064-022-03555-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03555-7