Abstract
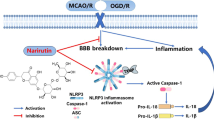
Intracerebral hemorrhage (ICH) is a devastating cerebrovascular disease with a high mortality rate affecting individuals worldwide. After ICH, persistent inflammation results in the death of brain cells, as well as the promotion of secondary brain injury. Verbascoside (VB), an active component in herbal medicine, possesses antioxidant, anti-inflammatory and neuroprotective properties. Furthermore, previous studies have shown that VB improves recovery of neuronal function after spinal cord injury in rats. In this study, we investigated whether VB limited inflammation induced by ICH through the targeting of NLRP3, which is associated with acute inflammation and apoptosis. Administration of VB reduced neurological impairment and pathological abnormalities associated with ICH, while increasing cell viability of neurons. This was achieved through NLRP3 inhibition and microglial activation. VB treatment decreased neuronal damage when co-cultured with microglia. Furthermore, knockout of NLRP3 eliminated the ability of VB to inhibit inflammation, cell death or protect neurons. Taken together, VB suppressed the inflammatory response following ICH by inhibiting NLRP3.




Similar content being viewed by others
References
Wang M, Cheng L, Chen ZL, Mungur R, Xu SH, Wu J, Liu XL, Wan S (2019) Hyperbaric oxygen preconditioning attenuates brain injury after intracerebral hemorrhage by regulating microglia polarization in rats. CNS Neurosci Ther 25:1126–1133
An SJ, Kim TJ, Yoon BW (2017) Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke 19:3–10
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5:53–63
Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurogibol 92:463–477
Wang J, Dore S (2007) Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab 27:894–908
Haque ME, Gabr RE, George SD, Zhao X, Boren SB, Zhang X, Ting SM, Sun G, Hasan KM, Savitz S, Aronowski J (2019) Serial metabolic evaluation of perihematomal tissues in the intracerebral hemorrhage pig model. Front Neurosci 13:888–889
Bobinger T, Burkardt P, Huttner HB, Manaenko A (2018) Programmed cell death after intracerebral hemorrhage. Curr Neuropharmacol 16:1267–1281
Rodríguez C, Sobrino T, Agulla J, Bobo-Jiménez V, Ramos-Araque ME, Duarte JJ, Gómez-Sánchez JC, Bolaños JP, Castillo J, Almeida Á (2017) Neovascularization and functional recovery after intracerebral hemorrhage is conditioned by the Tp53 Arg72Pro single-nucleotide polymorphism. Cell Death Differ 24:144–154
Salman RAS, Law ZK, Bath PM, Steiner T, Sprigg N (2018) Haemostatic therapies for acute spontaneous intracerebral haemorrhage. Cochrane Database Syst Rev 4:D5951
Wen J, Yang CY, Lu J, Wang XY (2018) Ptprj-as1 mediates inflammatory injury after intracerebral hemorrhage by activating NF-κB pathway. Eur Rev Med Pharmacol 22:2817–2823
Zhang Y, Yi B, Ma J, Zhang L, Zhang H, Yang Y, Dai Y (2015) Quercetin promotes neuronal and behavioral recovery by suppressing inflammatory response and apoptosis in a rat model of intracerebral hemorrhage. Neurochem Res 40:195–203
Xu J, Wang H, Ding K, Zhang L, Wang C, Li T, Wei W, Lu X (2014) Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2–ARE pathway. Free Radic Biol Med 71:186–195
Zhu HT, Bian C, Yuan JC, Chu WH, Xiang X, Chen F, Wang CS, Feng H, Lin JK (2014) Curcumin attenuates acute inflammatory injury by inhibiting the tlr4/myd88/nf-κb signaling pathway in experimental traumatic brain injury. J Neuroinflammation 59:1–17
Fann DY, Lee SY, Manzanero S, Chunduri P, Sobey CG, Arumugam TV (2013) Pathogenesis of acute stroke and the role of inflammasomes. Ageing Res Rev 12:941–966
Xu F, Shen G, Su Z, He Z, Yuan L (2019) Glibenclamide ameliorates the disrupted blood–brain barrier in experimental intracerebral hemorrhage by inhibiting the activation of NLRP3 inflammasome. Brain Behav 9:e01254
Ismael S, Zhao L, Nasoohi S, Ishrat T (2018) Inhibition of the NLRP3-inflammasome as a potential approach for neuroprotection after stroke. Sci Rep 8:5971
Lai X, Xiong Y, Zhou J, Yang F, Peng J, Chen L, Zhong W (2019) Verbascoside attenuates acute inflammatory injury in experimental cerebral hemorrhage by suppressing TLR4. Biochem Biophys Res Commun 519:721–726
Wang YC, Wang PF, Fang H, Chen J, Xiong XY, Yang QW (2013) Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 44:2545–2552
Xiong L, Mao S, Lu B, Yang J, Zhou F, Hu Y, Jiang Y, Shen C, Zhao Y (2016) Osmanthus fragrans flower extract and acteoside protect against d-galactose-induced aging in an icr mouse model. J Med Food 19:54–61
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats: statistical validation. Stroke 26:627–635
Yang Y, Zhang Y, Wang Z, Wang S, Gao M, Xu R, Liang C, Zhang H (2016) Attenuation of acute phase injury in rat intracranial hemorrhage by cerebrolysin that inhibits brain edema and inflammatory response. Neurochem Res 41:748–757
Chang CF, Wan J, Li Q, Renfroe SC, Heller NM, Wang J (2017) Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol Dis 103:54–69
Song HL, Zhang SB (2019) Therapeutic effect of dexmedetomidine on intracerebral hemorrhage via regulating NLRP3. Eur Rev Med Pharmacol Sci 23:2612–2619
Yuan R, Fan H, Cheng S, Gao W, Xu X, Lv S, Ye M, Wu M, Zhu X, Zhang Y (2017) Silymarin prevents NLRP3 inflammasome activation and protects against intracerebral hemorrhage. Biomed Pharmacother 93:308–315
Zhao H, Pan P, Yang Y, Ge H, Chen W, Qu J, Shi J, Cui G, Liu X, Feng H, Chen Y (2017) Endogenous hydrogen sulphide attenuates NLRP3 inflammasome-mediated neuroinflammation by suppressing the P2X7 receptor after intracerebral haemorrhage in rats. J Neuroinflammation 14:163
Yin D, Zhou S, Xu X, Gao W, Li F, Ma Y, Sun D, Wu Y, Guo Q, Liu H, Han L, Wang Z, Wang Y, Zhang J (1698) Dexmedetomidine attenuated early brain injury in rats with subarachnoid haemorrhage by suppressing the inflammatory response: The TLR4/NF-κB pathway and the NLRP3 inflammasome may be involved in the mechanism. Brain Res 2018:1–10
Zheng B, Zhang S, Ying Y, Guo X, Li H, Xu L, Ruan X (2018) Administration of Dexmedetomidine inhibited NLRP3 inflammasome and microglial cell activities in hippocampus of traumatic brain injury rats. Biosci Rep 38:BSR20180892
Alipieva K, Korkina L, Orhan IE, Georgiev MI (2014) Verbascoside-a review of its occurrence, (bio) synthesis and pharmacological significance. Biotechnol Adv 32:1065–1076
Pérez-Barrón G, Avila-Acevedo JG, García-Bores AM, Montes S, García-Jiménez S, León-Rivera I, Rubio-Osornio M, Monroy-Noyola A (2015) Neuroprotective effect of buddleja cordata methanolic extract in the 1-methyl-4-phenylpyridinium parkinson’s disease rat model. J Nat Med 69:86–93
Wang S, Yao Q, Wan Y, Wang J, Huang C, Li D, Yang B (2019) Adiponectin reduces brain injury after intracerebral hemorrhage by reducing NLRP3 inflammasome expression. Int J Neurosci 22:1–8
Li X, Wang T, Zhang D, Li H, Shen H, Ding X, Chen G (2018) Andrographolide ameliorates intracerebral hemorrhage induced secondary brain injury by inhibiting neuroinflammation induction. Neuropharmacology 141:305–315
Acknowledgments
I would like to express my gratitude to all who contributed to this study. I would like to thank my supervisor, Changren Huang, who has offered valuable suggestions and helped design the experiments. Secondly, I would like to express my heartfelt gratitude to Huiyong Huang who provided strong advice when revising this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, H., Zhang, C. & Huang, C. Verbascoside Attenuates Acute Inflammatory Injury Caused by an Intracerebral Hemorrhage Through the Suppression of NLRP3. Neurochem Res 46, 770–777 (2021). https://doi.org/10.1007/s11064-020-03206-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03206-9