Abstract
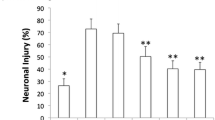
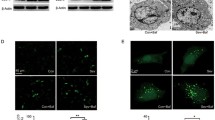
Hypoxic-ischemic brain injury (HIBI) in neonates is one of the major contributors of newborn death and cognitive impairment. Numerous animal studies have demonstrated that autophagy is substantially increased in HIBI and that sevoflurane postconditioning (SPC) can attenuate HIBI. However, if SPC-induced neuroprotection inhibits autophagy in HIBI remains unknown. To investigate if cerebral protection induced by SPC is related to decreased autophagy in the setting of HIBI. Postnatal rats at day 7 (P7) were randomly assigned to 7 different groups: Sham, HIBI, SPC–HIBI, HIBI + rapamycin, SPC–HIBI + rapamycin, HIBI + p-extracellular signal-regulated kinase (p-ERK) inhibitor, and SPC–HIBI + p-ERK inhibitor. To induce HIBI, neonatal rats underwent left common carotid artery ligation, followed by 2 h of hypoxia (8% O2). Rats in the SPC groups were treated with 1 minimum alveolar concentration ([MAC], 2.4%) SPC for 30 min after HIBI induction. Markers of autophagy and expression of ERK cascade components were measured in the rat brains after 24 h. Spatial learning and memory function were examined 29–34 days after administration of an autophagy agonist or a p-ERK inhibitor. The expression of microtubule-associated proteins 1A/1B, light chain 3B II (LC3-II) and tuberous sclerosis complex 2 (TSC2) were decreased in the SPC–HIBI group compared to the HIBI group. Expression of the p62 sequestosome 1 (P62/SQSTM1) protein, p-ERK/ERK, phospho-mammalian target of rapamycin (p-mTOR) and phospho-p70S6 were increased in SPC–HIBI group. Rats within the SPC–HIBI groups that also received the p-ERK inhibitor or autophagy inhibitor demonstrated reduced cross platform times and increased escape latency. Approximately 30 min of 2.4% SPC treatment in the P7 rat HIBI model attenuated excessive autophagy in the brain by elevating the ERK cascade. This finding provides additional insight into HIBI and identifies new targets for therapeutic approaches to treat HIBI.






Similar content being viewed by others
Abbreviations
- HIBI:
-
Hypoxic-ischemic brain injury
- SPC:
-
Sevoflurane postconditioning
- ERK:
-
Extracellular signal-regulated kinase
- ERKI:
-
ERK inhibitor
References
Edwards A, Brocklehurst P, Gunn A, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzopardi D (2010) Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 340:c363
Descloux C, Ginet V, Clarke PG, Puyal J, Truttmann AC (2015) Neuronal death after perinatal cerebral hypoxia-ischemia: Focus on autophagy-mediated cell death. Int J Dev Neurosci 45:75–85. https://doi.org/10.1016/j.ijdevneu.2015.06.008
Okereafor A, Allsop J, Counsell SJ, Fitzpatrick J, Azzopardi D, Rutherford MA, Cowan FM (2008) Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121(5):906–914. https://doi.org/10.1542/peds.2007-0770
Vannucci SJ, Hagberg H (2004) Hypoxia-ischemia in the immature brain. J Exp Biol 207(Pt 18):3149–3154. https://doi.org/10.1242/jeb.01064
Stankowski JN, Gupta R (2011) Therapeutic targets for neuroprotection in acute ischemic stroke: lost in translation? Antioxid Redox Signal 14(10):1841–1851. https://doi.org/10.1089/ars.2010.3292
Yuan J (2009) Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis 14(4):469–477. https://doi.org/10.1007/s10495-008-0304-8
Cotten CM, Murtha AP, Goldberg RN, Grotegut CA, Smith PB, Goldstein RF, Fisher KA, Gustafson KE, Waters-Pick B, Swamy GK, Rattray B, Tan S, Kurtzberg J (2014) Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr 164(5):973–979 e971. https://doi.org/10.1016/j.jpeds.2013.11.036
Puyal J, Ginet V, Clarke PG (2013) Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. Prog Neurobiol 105:24–48. https://doi.org/10.1016/j.pneurobio.2013.03.002
Kim H, Kim E, Bae J, Lee K, Jeon Y, Hwang J, Lim Y, Min S, Park H (2017) Sevoflurane postconditioning reduces apoptosis by activating the JAK-STAT pathway after transient global cerebral ischemia in rats. J Neurosurg Anesthesiol 29(1):37–45
Wang H, Shi H, Yu Q, Chen J, Zhang F, Gao Y (2016) Sevoflurane preconditioning confers neuroprotection via anti-apoptosis effects. Acta Neurochir Suppl 121:55–61. https://doi.org/10.1007/978-3-319-18497-5_10
Hwang J, Jeon Y, Lim Y, Park H (2017) Sevoflurane postconditioning-induced anti-inflammation via inhibition of the toll-like receptor-4/nuclear factor Kappa B pathway contributes to neuroprotection against transient global cerebral ischemia in rats. Int J Mol Sci 18(11):2347
Xu Y, Tian Y, Tian Y, Li X, Zhao P (2016) Autophagy activation involved in hypoxic-ischemic brain injury induces cognitive and memory impairment in neonatal rats. J Neurochem 139(5):795–805. https://doi.org/10.1111/jnc.13851
Shang L, Chen S, Du F, Li S, Zhao L, Wang X (2011) Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci USA 108(12):4788–4793. https://doi.org/10.1073/pnas.1100844108
von Kriegsheim A, Baiocchi D, Birtwistle M, Sumpton D, Bienvenut W, Morrice N, Yamada K, Lamond A, Kalna G, Orton R, Gilbert D, Kolch W (2009) Cell fate decisions are specified by the dynamic ERK interactome. Nat Cell Biol 11(12):1458–1464. https://doi.org/10.1038/ncb1994
Yuan JH, Pan F, Chen J, Chen CE, Xie DP, Jiang XZ, Guo SJ, Zhou J (2017) Neuroprotection by plumbagin involves BDNF-TrkB-PI3K/Akt and ERK1/2/JNK pathways in isoflurane-induced neonatal rats. J Pharm Pharmacol 69(7):896–906. https://doi.org/10.1111/jphp.12681
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23(3):876–882
Zhao P, Peng L, Li L, Xu X, Zuo Z (2007) Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology 107(6):963–970. https://doi.org/10.1097/01.anes.0000291447.21046.4d
Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA (2011) Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain 134(Pt 1):258–277. https://doi.org/10.1093/brain/awq341
Hu X, Wang J, Zhang Q, Duan X, Chen Z, Zhang Y (2016) Postconditioning with sevoflurane ameliorates spatial learning and memory deficit after hemorrhage shock and resuscitation in rats. J Surg Res 206(2):307–315
Lai Z, Zhang L, Su J, Cai D, Xu Q (2016) Sevoflurane postconditioning improves long-term learning and memory of neonatal hypoxia-ischemia brain damage rats via the PI3K/Akt-mPTP pathway. Brain Res 1630:25–37
Lawn J, Bahl R, Bergstrom S, Bhutta Z, Darmstadt G, Ellis M, English M, Kurinczuk J, Lee A, Merialdi M, Mohamed M, Osrin D, Pattinson R, Paul V, Ramji S, Saugstad O, Sibley L, Singhal N, Wall S, Woods D, Wyatt J, Chan K, Rudan I (2011) Setting research priorities to reduce almost one million deaths from birth asphyxia by 2015. PLoS Med 8(1):e1000389
Fineschi V, Viola RV, La Russa R, Santurro A, Frati P (2017) A controversial medicolegal issue: timing the onset of perinatal hypoxic-ischemic brain injury. Mediators Inflamm 2017:6024959. https://doi.org/10.1155/2017/6024959
Wang Z, Ye Z, Huang G, Wang N, Wang E, Guo Q (2016) Sevoflurane post-conditioning enhanced hippocampal neuron resistance to global cerebral ischemia induced by cardiac arrest in rats through PI3K/Akt survival pathway. Front Cell Neurosci 10:271. https://doi.org/10.3389/fncel.2016.00271
Ginet V, Puyal J, Clarke PG, Truttmann AC (2009) Enhancement of autophagic flux after neonatal cerebral hypoxia-ischemia and its region-specific relationship to apoptotic mechanisms. Am J Pathol 175(5):1962–1974. https://doi.org/10.2353/ajpath.2009.090463
Piras A, Gianetto D, Conte D, Bosone A, Vercelli A (2011) Activation of autophagy in a rat model of retinal ischemia following high intraocular pressure. PLoS ONE 6(7):e22514. https://doi.org/10.1371/journal.pone.0022514
Ginet V, Spiehlmann A, Rummel C, Rudinskiy N, Grishchuk Y, Luthi-Carter R, Clarke PG, Truttmann AC, Puyal J (2014) Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy 10(5):846–860. https://doi.org/10.4161/auto.28264
Liu Y, Levine B (2015) Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ 22(3):367–376
Puyal J, Ginet V, Grishchuk Y, Truttmann AC, Clarke PG (2012) Neuronal autophagy as a mediator of life and death: contrasting roles in chronic neurodegenerative and acute neural disorders. Neuroscientist 18(3):224–236. https://doi.org/10.1177/1073858411404948
Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, Hagberg H, Blomgren K (2005) The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ 12(2):162–176. https://doi.org/10.1038/sj.cdd.4401545
Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY (2006) Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol 169(2):566–583. https://doi.org/10.2353/ajpath.2006.051066
Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH (2008) Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 4(6):762–769
Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y (2008) Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 172(2):454–469. https://doi.org/10.2353/ajpath.2008.070876
Puyal J, Clarke P (2009) Targeting autophagy to prevent neonatal stroke damage. Autophagy 5(7):1060–1061
Puyal J, Vaslin A, Mottier V, Clarke P (2009) Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol 66(3):378–389
Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z (2010) LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 29(11):1792–1802. https://doi.org/10.1038/emboj.2010.74
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3(6):542–545
Kovács V, Tóth-Szűki V, Németh J, Varga V, Remzső G, Domoki F (2018) Active forms of Akt and ERK are dominant in the cerebral cortex of newborn pigs that are unaffected by asphyxia. Life Sci 192:1–8
Egan D, Kim J, Shaw RJ, Guan KL (2011) The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 7(6):643–644
Kim SY, Cheon SY, Kim EJ, Lee JH, Kam EH, Kim JM, Park M, Koo BN (2017) Isoflurane postconditioning inhibits tPA-induced matrix metalloproteinases activation after hypoxic injury via low-density lipoprotein receptor-related protein and extracellular signal-regulated kinase pathway. Neurochem Res 42(5):1533–1542. https://doi.org/10.1007/s11064-017-2211-2
Funding
This study was supported by two grants from the National Natural Science Foundation of China (Nos. 81171782, 81671311), one grant from Science and Technology foundation of Liaoning province (No. 2015020467), one grant from the Outstanding Scientific Fund of Shengjing Hospital (No. 201708).
Author information
Authors and Affiliations
Contributions
SW and PZ designed the experiments. SW, HX and PZ contributed to the planning of the work. SW performed all the experiments with the help of HX, YX and JN. HX, YX and JN participated in the data collection. SW, YX and JN analyzed and interpreted the results. SW wrote the manuscript with the help of YX. PZ supervised the project and revised the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics Approval
All animal experiments were carried out in accordance with the National Institute of Health Guideline for the Care and Use of Laboratory Animals. Formal approval to conduct the experiments described has been obtained from the animal review board of Shengjing Hospital, China Medical University.
Rights and permissions
About this article
Cite this article
Wang, S., Xue, H., Xu, Y. et al. Sevoflurane Postconditioning Inhibits Autophagy Through Activation of the Extracellular Signal-Regulated Kinase Cascade, Alleviating Hypoxic-Ischemic Brain Injury in Neonatal Rats. Neurochem Res 44, 347–356 (2019). https://doi.org/10.1007/s11064-018-2682-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2682-9