Abstract
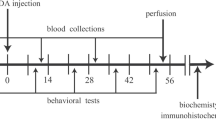
Epidemiological studies indicate that a higher plasma level of uric acid (UA) associates with the reduced risk of Parkinson’s disease (PD). To confirm the role of UA as a biomarker for PD, we evaluated changes in the serum UA level in the 6-hydroxydopamine (6-OHDA)-induced hemiparkinsonism in rat. For this purpose, 6-OHDA was administered in the medial forebrain bundle by stereotaxic surgery. According to the apomorphine-induced rotational test, the increased intensity of behavioral symptoms as a function of time was associated with the further reduction of UA level. On the other hand, the level of UA increased in the midbrain of the injured hemisphere. The level of reduction in the serum UA level of rats with severe and moderate symptoms was significantly higher than that of rats with mild symptoms. The immunohistofluorescence and biochemical analyses showed that the serum UA level was also correlated with the death of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra pars compacta (SNc), reduced level of striatal dopamine, and severity of oxidative stress in the midbrain. The rats with mild symptoms also showed a significant decrease in TH-positive neurons and striatal dopamine level. These findings suggest a positive correlation between the level of reduction in the serum urate level and severity of 6-OHDA-induced Parkinsonism. In addition, our findings indicated that UA had no marked neuroprotective effects, at least at concentrations obtained in this study. On the other hand, UA was introduced as a biomarker for PD, as a significant decline was observed in the serum UA level of rats with mild behavioral symptoms but with significant dopaminergic cell death in the SNc.





Similar content being viewed by others
References
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373(9680):2055–2066. https://doi.org/10.1016/S0140-6736(09)60492-X
Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM (2007) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68(5):384–386
Moon HE, Paek SH (2015) Mitochondrial dysfunction in Parkinson’s disease. Exp Neurobiol 24:103–116. https://doi.org/10.5607/en.2015.24.2.103
Tsang AHK, Chung KKK (2009) Oxidative and nitrosative stress in Parkinson’s disease. Biochem Biophys Acta 1792:643–650. https://doi.org/10.1016/j.bbadis.2008.12.006
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909. https://doi.org/10.1016/S0896-6273(03)00568-3
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurol 47:S161–S170
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222. https://doi.org/10.1146/annurev-pathol-011110-130242
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. https://doi.org/10.1136/jnnp.2007.131045
Frasier M, Chowdhury S, Eberling J, Sherer T (2010) Biomarkers in Parkinson’s disease: a funder’s perspective. Biomark Med 4(5):723–729. https://doi.org/10.2217/bmm.10.89
Ames BN, Cathcart R, Schwiers E, Hochstein P (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 78(11):6858–6862
Yeum KJ, Russell RM, Krinsky NI, Aldini G (2004) Biomarkers of antioxidant capacity in the hydrophilic and lipophilic compartments of human plasma. Arch Biochem Biophys 430(1):97–103. https://doi.org/10.1016/j.abb.2004.03.006
Church WH, Ward VL (1994) Uric acid is reduced in the substantia nigra in Parkinson’s disease: effect on dopamine oxidation. Brain Res Bull 33(4):419–425
Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM (1996) Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol 144(5):480–484
de Lau LM, Koudstaal PJ, Hofman A, Breteler MM (2005) Serum uric acid levels and the risk of Parkinson disease. Ann Neurol 58(5):797–800. https://doi.org/10.1002/ana.20663
Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A (2007) Plasma urate and risk of Parkinson’s disease. Am J Epidemiol 166(5):561–567. https://doi.org/10.1093/aje/kwm127
Alonso A, Rodríguez LA, Logroscino G, Hernán MA (2007) Gout and risk of Parkinson disease: a prospective study. Neurology 69(17):1696–1700. https://doi.org/10.1212/01.wnl.0000279518.10072.df
Chen H, Mosley TH, Alonso A, Huang X (2009) Plasma urate and Parkinson’s disease in the Atherosclerosis Risk in Communities (ARIC) study. Am J Epidemiol 169(9):1064–1069. https://doi.org/10.1093/aje/kwp033
Schwarzschild MA, Macklin EA, Ascherio A (2014) Parkinson Study Group SURE-PD Investigators: urate and neuroprotection trials. Lancet Neurol 13(8):758. https://doi.org/10.1016/S1474-4422(14)70138-3
De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H (2008) Gout and the risk of Parkinson’s disease: a cohort study. Arthritis Rheum 59(11):1549–1554. https://doi.org/10.1002/art.24193
Annanmaki T, Muuronen A, Murros K (2007) Low plasma uric acid level in Parkinson’s disease. Mov Disord 15(8):1133–1137
Vieru E, Köksal A, Mutluay B, Dirican AC, Altunkaynak Y, Baybas S (2016) The relation of serum uric acid levels with L-Dopa treatment and progression in patients with Parkinson’s disease. Neurol Sci 37(5):743–747. https://doi.org/10.1007/s10072-015-2471-z
Andreadou E, Nikolaou C, Gournaras F, Rentzos M, Boufidou F, Tsoutsou A, Zournas C, Zissimopoulos V, Vassilopoulos D (2009) Serum uric acid levels in patients with Parkinson’s disease: their relationship to treatment and disease duration. Clin Neurol Neurosurg 111(9):724–728. https://doi.org/10.1016/j.clineuro.2009.06.012
Bové J, Perier C (2012) Neurotoxin-based models of Parkinson’s disease. Neuroscience 211:51–76. https://doi.org/10.1016/j.neuroscience.2011.10.057
Blandini F, Armentero MT, Martignoni E (2008) The 6-ydroxydopamine model: news from the past. Parkinsonism Relat Disord 14(Suppl 2):S124–S129. https://doi.org/10.1016/j.parkreldis.2008.04.015
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, San Diego
Fujita M, Nishino H, Kumazaki M, Shimada S, Tohyama M, Nishimura T (1996) Expression of dopamine transporter mRNA and its binding site in fetal nigral cells transplanted into the striatum of 6-OHDA lesioned rat. Mol Brain Res 39:127–136
Sarookhani MR, Haghdoost-Yazdi H, Sarbazi-Golezari A, Babayan-Tazehkand A, Rastgoo N (2017) Involvement of adenosine triphosphate -sensitive potassium channels in the neuroprotective activity of hydrogen sulfide in the 6-hydroxydopamine- induced animal model of Parkinson’s disease. Behav Pharmacol. https://doi.org/10.1097/FBP.0000000000000358
Sarukhani M, Haghdoost-Yazdi H, Sarbazi A, Babayan-Tazehkand A, Dargahi T, Rastgoo N (2018) Evaluation of the antiparkinsonism and neuroprotective effects of hydrogen sulfide in acute 6-hydroxydopamine- induced animal model of Parkinson’s disease: behavioral, histological and biochemical studies. Neurol Res (unpublished data)
Zhang N, Shu HY, Huang T, Zhang QL, Li D, Zhang GQ, Peng XY, Liu CF, Luo WF, Hu LF (2014) Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS ONE 9(6):e100286. https://doi.org/10.1371/journal.pone.0100286
Zhu TG, Wang XX, Luo WF, Zhang QL, Huang TT, Xu XS, Liu CF (2012) Protective effects of urate against 6-OHDA-induced cell injury in PC12 cells through antioxidant action. Neurosci Lett 506(2):175–179. https://doi.org/10.1016/j.neulet.2011.10.075
Gong L, Zhang QL, Zhang N, Hua WY, Huang YX, Di PW, Huang T, Xu XS, Liu CF, Hu LF, Luo WF (2012) Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: linking to Akt/GSK3β signaling pathway. J Neurochem 123(5):876–885. https://doi.org/10.1111/jnc.12038
De Luca MA, Cauli O, Morelli M, Simola N (2014) Elevation of striatal urate in experimental models of Parkinson’s disease: a compensatory mechanism triggered by dopaminergic nigrostriatal degeneration? J Neurochem 131(3):284–289. https://doi.org/10.1111/jnc.12809
Fitzmaurice PS, Ang L, Guttman M, Rajput AH, Furukawa Y, Kish SJ (2003) Nigral glutathione deficiency is not specific for idiopathic Parkinson’s disease. Mov Disord 18(9):969–976. https://doi.org/10.1002/mds.10486
Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF (2010) Uric acid as a CNS antioxidant. J Alzheimers Dis 19(4):1331–1336. https://doi.org/10.3233/JAD-2010-1330
Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD (2005) 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci 22(5):1158–1168. https://doi.org/10.1111/j.1460-9568.2005.04281.x
Cooper PH, Novin D, Butcher LL (1982) Intracerebral 6-hydroxydopamine produces extensive damage to the blood-brain barrier in rats. Neurosci Lett 30(1):13–18
Paganoni S, Schwarzschild MA (2017) Urate as a marker of risk and progression of neurodegenerative disease. Neurotherapeutics 14(1):148–153. https://doi.org/10.1007/s13311-016-0497-4
Wen M, Zhou B, Chen YH, Ma ZL, Gou Y, Zhang CL, Yu WF, Jiao L (2017) Serum uric acid levels in patients with Parkinson’s disease: a meta-analysis. PLoS ONE 12(3):e0173731. https://doi.org/10.1371/journal.pone.0173731
Yuan H, Sarre S, Ebinge G, Michotte Y (2005) Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J Neurosci Methods 144:35–45. https://doi.org/10.1016/j.jneumeth.2004.10.004
Abrous DN, Rodriguez JJ, Montaron MF, Aurousseau C, Le Moal M, Barneoud P (1998). Behavioural recovery after unilateral lesion of the dopaminergic mesotelencephalic pathway: effect of repeated testing. Neurosci 84:213–221
Iancu R, Mohapel P, Brundin P, Paul G (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162:1–10. https://doi.org/10.1016/j.bbr.2005.02.023
Acknowledgements
We would like to thank Mrs. Ayda Faraji for her assistance in stereotaxic surgeries and Ms. Zare for her contribution to immunohistofluorescence experiments. This study was supported by a Grant-in-aid for scientific research from the Research Council of Qazvin University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sarukhani, M.R., Haghdoost-Yazdi, H. & Khandan-Chelarci, G. Changes in the Serum Urate Level Can Predict the Development of Parkinsonism in the 6-Hydroxydopamine Animal Model. Neurochem Res 43, 1086–1095 (2018). https://doi.org/10.1007/s11064-018-2522-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-018-2522-y