Abstract
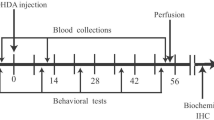
Mild to moderate dopaminergic (DA) neuronal death in substantia nigra pars compacta (SNc) as the main pathological hallmark of Parkinson’s disease (PD) is usually silent and does not produce marked clinical symptoms. In this study, we investigated the association between SNc DA neuronal loss and serum levels of total bilirubin (TB), selenium (Se), and zinc (Zn) in 6-hydroxydopamine (6-OHDA) animal model of PD. The neurotoxin of 6-OHDA was injected into the medial forebrain bundle of right hemisphere by stereotaxic surgery. Two conventional behavioral tests were carried out in several steps after the toxin to confirm the model reproduction and quantify severity and progress of 6-OHDA-induced PD. Blood samples were collected within 1 week before the toxin and in the second, fifth, and eighth weeks thereafter. Immunohistochemistry (IHC) assessments were performed on the rat’s brain to determine the severity of DA neuronal loss in SNc. The severity of behavioral symptoms and TB levels were progressively increased in 6-OHDA-treated rats. On the other hand, Se and Zn levels in them were lower than control. These changes were observed in rats with severe or mild behavioral symptoms. Also, IHC revealed that changes in TB, Se, and Zn associate with SNc DA neuronal loss but do not correlate with its severity. Significant changes in serum levels of TB, Se, and Zn in the mild SNc DA neuronal loss suggest them as valuable parameters for establishment of a serum profile for early detection of PD.





Similar content being viewed by others
Data Availability
The datasets used during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Tsang AHK, Chung KKK (2009) Oxidative and nitrosative stress in Parkinson’s disease. Biochem Biophys Acta 1792:643–650. https://doi.org/10.1016/j.bbadis.2008.12.006
Raza C, Anjum R, Shakeel NUA (2019) Parkinson’s disease: mechanisms, translational models and management strategies. Life Sci 226:77–90. https://doi.org/10.1016/j.lfs.2019.03.057
Dorszewska J, Kowalska M, Prendecki M et al (2021) Oxidative stress factors in Parkinson’s disease. Neural Regen Res 16(7):1383–1391. https://doi.org/10.4103/1673-5374.300980
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376. https://doi.org/10.1136/jnnp.2007.131045
Shulman JM, De Jager PL, Feany MB (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222. https://doi.org/10.1146/annurev-pathol-011110-130242
Sedlak TW, Saleh M, Higginson DS et al (2009) Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A 106(13):5171–5176. https://doi.org/10.1073/pnas.0813132106
Wu TW, Fung KP, Yang CC (1994) Unconjugated bilirubin inhibits the oxidation of human low density lipoprotein better than Trolox. Life Sci 54(25):477–481. https://doi.org/10.1016/0024-3205(94)90140-6
Soto Conti CP (2021) Bilirubin: the toxic mechanisms of an antioxidant molecule. J Atheroscler Thromb 26(8):688–696. https://doi.org/10.5551/jat.RV17035
Fujiwara R, Haag M, Schaeffeler E et al (2018) Systemic regulation of bilirubin homeostasis: potential benefits of hyperbilirubinemia. Hepatology 67(4):1609–1619. https://doi.org/10.1002/hep.29599
Schipper HM, Song W, Zukor H et al (2009) Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 110(2):469–485. https://doi.org/10.1111/j.1471-4159.2009.06160.x
McCarty MF (2013) Serum bilirubin may serve as a marker for increased heme oxygenase activity and inducibility in tissues—a rationale for the versatile health protection associated with elevated plasma bilirubin. Med Hypotheses 81(4):607–610. https://doi.org/10.1016/j.mehy.2013.07.013
Jin J-N, Liu X, Li M-J et al (2020) Association between serum bilirubin concentration and Parkinson’s disease: a meta-analysis. Chin Med J 134(6):655–661. https://doi.org/10.1097/CM9.0000000000001300
Ellwanger JH, Franke SIR, Bordin DL et al (2016) Biological functions of selenium and its potential influence on Parkinson’s disease. An Acad Bras Cienc 88:1655–1674
Maassa F, Michalke B, Willkommen D et al (2010) Selenium speciation analysis in the cerebrospinal fluid of patients with Parkinson’s disease. Trace Elem Med Biol 57:110–115. https://doi.org/10.1016/j.jtemb.2019.126412
Castaño A, Ayala A, Rodrı́guez-Gómez JA, et al (1997) Low selenium diet increases the dopamine turnover in prefrontal cortex of the rat. Neurochem Int 30(6):549–555. https://doi.org/10.1016/s0197-0186(96)00123-4
Sun H, Liu X, Ge H et al (2017) Association between serum zinc levels and the risk of Parkinson’s disease: a meta-analysis. Biol Trace Elem Res 179:45–51. https://doi.org/10.1007/s12011-017-0941-2
Barmaki H, Morovati A, Eydivandi Z et al (2021) The association between serum oxidative stress indexes and pathogenesis of Parkinson’s disease in the northwest of Iran. Iran J Public Health 50(3):606–615. https://doi.org/10.18502/ijph.v50i3.5621
Qin XL, Zhang QS, Sun L et al (2015) Lower serum bilirubin and uric acid concentrations in patients with Parkinson’s disease in China. Cell Biochem Biophys 72:49–56. https://doi.org/10.1007/s12013-014-0402-x
Scigliano G, Girotti F, Soliveri P et al (1997) Increased plasma bilirubin in Parkinson patients on L-dopa: evidence against the free radical hypothesis? Ital J Neurol Sci 18(2):69–72. https://doi.org/10.1007/BF01999565
Moccia M, Picillo M, Erro R et al (2015) Increased bilirubin levels in de novo Parkinson’s disease. Eur J Neurol 22(6):954–959. https://doi.org/10.1111/ene.12688
Macias-Garcia D, Mendez-Del Barrio C, Jesus S, Labrador MA et al (2019) Increased bilirubin levels in Parkinson’s disease. Parkinson Relat Disord 63:213–216. https://doi.org/10.1016/j.parkreldis.2019.01.012
Hernandez-Baltazar DH, Zavala-Flores LM, Villanueva-Olivo A (2017) The 6-hydroxydopamine model and parkinsonian pathophysiology: novel findings in an older model. Neurologia 32(8):533–539. https://doi.org/10.1016/j.nrl.2015.06.011
Sarukhani MR, Haghdoost-Yazdi H, Khandan-Chelarci G (2018) Changes in the serum urate level can predict the development of Parkinsonism in the 6-hydroxydopamine animal model. Neurochem Res 43(5):1086–1095. https://doi.org/10.1007/s11064-018-2522-y
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, San Diego
Sarukhani M, Haghdoost-Yazdi H, Sarbazi Golezari A et al (2018) Evaluation of the antiparkinsonism and neuroprotective effects of hydrogen sulfide in acute 6-hydroxydopamine-induced animal model of Parkinson’s disease: behavioral, histological and biochemical studies. Neurol Res 1–9. https://doi.org/10.1080/01616412.2017.1390903
Schallert T, Kozlowski DA, Humm JL et al (1997) Use-dependent structural events in recovery of function. Adv Neurol 73:229–38 (Review)
Songsomboon C, Tanprawate S, Soontornpun A et al (2020) Serum uric acid, serum uric acid to serum creatinine ratio and serum bilirubin in patients with Parkinson’s disease: a case-control study. J Clin Med Res 12:172–179. https://doi.org/10.14740/jocmr4079
Hatano T, Saiki S, Okuzumi A et al (2016) Identification of novel biomarkers for Parkinson’s disease by metabolomic technologies. J Neurol Neurosurg Psychiatry 87:295–301. https://doi.org/10.1136/jnnp-2014-309676
Li J, Zhao L, Wang Z et al (2019) Association of serum indirect bilirubin concentrations with motor subtypes of Parkinson’s disease. Neurodegener Dis 19:155–161. https://doi.org/10.1159/000505852
Ayuso P, Martínez C, Pastor P et al (2014) An association study between heme oxygenase-1 genetic variants and Parkinson’s disease. Front Cell Neurosci 8:298. https://doi.org/10.3389/fncel.2014.00298
Iancu R, Mohapel P, Brundin P et al (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162(1):1–10. https://doi.org/10.1016/j.bbr.2005.02.023
Yuan H, Sarre S, Ebinger G et al (2005) Histological, behavioral and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J Neurosci Methods 144(1):35–45. https://doi.org/10.1016/j.jneumeth.2004.10.004
Hasuike Y, Endo T, Koroyasu M et al (2020) Bile acid abnormality induced by intestinal dysbiosis might explain lipid metabolism in Parkinson’s disease. Med Hypotheses 134:109436. https://doi.org/10.1016/j.mehy.2019.109436
Minaei A, Haghdoost-Yazdi H (2019) Dexmedetomidine attenuates the induction and reverses the progress of 6-hydroxydopamine-induced parkinsonism; involvement of KATP channels, alpha 2 adrenoceptors and anti-inflammatory mechanisms. Toxicol Appl Pharmacol 382:114743. https://doi.org/10.1016/j.taap.2019.114743
Minaei A, Sarookhani MR, Haghdoost-Yazdi H et al (2021) Hydrogen sulfide attenuates induction and prevents progress of the 6-hydroxydopamine-induced Parkinsonism in rat through activation of ATP-sensitive potassium channels and suppression of ER stress. Toxicol Appl Pharmacol 423:115558. https://doi.org/10.1016/j.taap.2021.115558
Zhang X, Liu RP, Cheng WH et al (2019) Prioritized brain selenium retention and selenoprotein expression: nutritional insights into Parkinson’s disease. Mech Ageing Dev 180:89–96. https://doi.org/10.1016/j.mad.2019.04.004
Ooi TC, Mohammad NH, Sharif R (2014) Zinc carnosine protects against hydrogen peroxide-induced DNA damage in WIL2-NS lymphoblastoid cell line independent of poly (ADP-ribose) polymerase expression. Biol Trace Elem Res 162(1–3):8–17. https://doi.org/10.1007/s12011-014-0153-y29
Kara E, Gunay M, Cicioglu I et al (2010) Effect of zinc supplementation on antioxidant activity in young wrestlers. Biol Trace Elem Res 134(1):55–63. https://doi.org/10.1007/s12011-009-8457-z30
Eide DJ (2011) The oxidative stress of zinc deficiency. Metallomics 3(11):1124–1129. https://doi.org/10.1039/c1mt00064k
Wang Z, Yang Y, Xiang X et al (2010) Estimation of the normal range of blood glucose in rats. 39(2):133–7, 142
Chan YK, Davis PF, Poppitt SD et al (2012) Influence of tail versus cardiac sampling on blood glucose and lipid profiles in mice. Lab Anim 46(2):142–147. https://doi.org/10.1258/la.2011.011136
Seibel J, Bodié K, Weber S et al (2010) Comparison of haematology, coagulation and clinical chemistry parameters in blood samples from the sublingual vein and vena cava in Sprague-Dawley rats. Lab Anim 44(4):344–351. https://doi.org/10.1258/la.2010.009049
Uemura K, Shintani-Ishida K, Saka K et al (2008) Biochemical blood markers and sampling sites in forensic autopsy. J Forensic Leg Med 15(5):312–317. https://doi.org/10.1016/j.jflm.2007.12.003
Chauhan A (2006) Pandey SK (2006) Haemato-biochemical effects of epidural fentanyl-ketamine combinations in dogs. J Bombay Vet Coll 14:96–99
Acknowledgements
Authors thank Miss Minaei for her assistance in immunohistochemistry studies and also Mrs. Dargahi for her assistance in blood collections.
Funding
This study was supported by a grant-in-aid for scientific research from the Research Council of Qazvin University of Medical Sciences (grant number: IR.QUMS.REC.1397.083).
Author information
Authors and Affiliations
Contributions
Study was designed by Haghdoost-Yazdi H and Sophiabadi M. Blood sampling and measurements of the bilirubin, Se, and Zn were managed by Rastgoo N. All authors involved in modeling of the animals and immunohistochemical assessments. Data was analyzed by Rastgoo N and manuscript wrote by all authors.
Corresponding author
Ethics declarations
Ethics Approval
All procedures of the present study were carried out according to the guidelines of animal experiments of the Research Council at Qazvin University of Medical Sciences. All authors confirm all data presented in this manuscript and consent for its submission in Biological Trace Element Research.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sophiabadi, M., Rastgoo, N. & Haghdoost-Yazdi, H. Dopaminergic Neuronal Death in Substantia Nigra Associates with Serum Levels of Total Bilirubin, Selenium, and Zinc: Evidences from 6-Hydroxydopamine Animal Model of Parkinson’s Disease. Biol Trace Elem Res 200, 4058–4067 (2022). https://doi.org/10.1007/s12011-021-03012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03012-6