Abstract
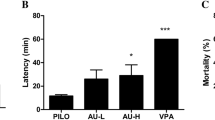
Epilepsy is one of the common and major neurological disorders, approximately a third of the individuals with epilepsy suffer from seizures and not able to successfully respond to available medications. Current study was designed to investigate whether Swertiamarin (Swe) had anticonvulsant activity in the pilocarpine (PILO)-treated mice. Thirty minutes prior to the PILO (280 mg/kg) injection, the mice were administrated with Swe (50, 150, and 450 mg/kg) and valproate sodium (VPA, 200 mg/kg) once. Seizures and electroencephalography (EEG) were observed, and then the mice were killed for Nissl, Fluoro-jade B (FJB) staining. Astrocytic activation was examined in the hippocampus. Western blot analysis was used to examine the expressions of interleukin-1β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α) and interleukin-10 (IL-10). The results indicated that pretreatment with Swe (150, 450 mg/kg) and VPA (200 mg/kg) significantly delayed the onset of the first convulsion and reduced the incidence of status epilepticus and mortality. Analysis of EEG recordings demonstrated that Swe (150, 450 mg/kg) and VPA (200 mg/kg) sharply decreased epileptiform discharges. Furthermore, Nissl and FJB staining revealed that Swe (150, 450 mg/kg) and VPA (200 mg/kg) relieved the neuronal damage. Additionally, Swe (450 mg/kg) dramatically inhibited astrocytic activation. Western blot analysis showed that Swe (450 mg/kg) significantly decreased the expressions of IL-1β, IL-6, TNF-α and elevated the expression of IL-10. Taken together, these findings revealed that Swe exerted anticonvulsant effects on PILO-treated mice. Further studies are encouraged to investigate these beneficial effects of Swe as an adjuvant in epilepsy.






Similar content being viewed by others
References
Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J Jr (2005) Epileptic seizures and epilepsy-definitions proposed by the international league against epilepsy (ILEA) and the international bureau for epilepsy (IBE). Epilepsia 46:470–472
Coelho VR, Vieira CG et al (2015) Antiepileptogenic, antioxidant and genotoxic evaluation of rosmarinic acid and its metabolite caffeic acid in mice. Life Sci 122:65–71
Dalic L, Cook MJ (2016) Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr Dis Treat 12:2605–2616
Simon RP, Greenberg DA, Aminof MJ (2009) Clinical neurology, 7th edn. McGraw-Hill, New York
Löscher W (2017) Animal models of seizures and epilepsy: past, present, and future role for the discovery of antiseizure drugs. Neurochem Res. doi:10.1007/s11064-017-2222-z [Epub ahead of print]
Castro OW, Furtado MA, Tilelli CQ et al (2010) Comparative neuroanatomical and temporal characterization of fluoro-jade-positive neurodegeneration after status epilepticus induced by systemic and intrahippocampal pilocarpine in wistar rats. Brain Res 1374:43–55
Shapiro LA, Wang L, Ribak CE (2008) Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia 49:33–41
Vezzani A, Dingledine R, Rossetti AO (2015) Immunity and inflammation in status epilepticus and its sequelae: possibilities for therapeutic application. Expert Rev Neurother 15:1081–1092
Vezzani A, Lang B, Aronica E (2015) Immunity and inflammation in epilepsy. Cold Spring Harb Perspect Med 6(2):a022699
Dambach H, Hinkerohe D, Prochnow N, Stienen MN, Moinfar Z, Haase CG, Hufnagel A, Faustmann PM (2014) Glia and epilepsy: experimental investigation of antiepileptic drugs in an astroglia/microglia co-culture model of inflammation. Epilepsia 55(1):184–192
Robel S, Sontheimer H (2016) Glia as drivers of abnormal neuronal activity. Nat Neurosci 19:28–33
Shen Y, Qin H, Chen J et al (2016) Postnatal activation of TLR4 in astrocytes promotes excitatory synaptogenesis in hippocampal neurons. J Cell Biol 215:719–734
Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to cns damage and disease. Neuron 81:229–248
Aronica E, Crino PB (2011) Inflammation in epilepsy: clinical observations. Epilepsia 52:26–32
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Vezzani A, Balosso S, Ravizza T (2012) Inflammation and epilepsy. Handb Clin Neurol 107:163–175
Vezzani A, Friedman A, Dingledine RJ (2013) The role of inflammation in epileptogenesis. Neuropharmacology 69:16–24
Jiang J, Yang MS, Quan Y, Gueorguieva P, Ganesh T, Dingledine R (2015) Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol Dis 76:126–136
Du Y, Kemper T, Qiu J, Jiang J (2016) Defining the therapeutic time window for suppressing the inflammatory prostaglandin E2 signaling after status epilepticus. Expert Rev Neurother 16:123–130
Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, Fitz Gerald GA, Dingledine R (2011) Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci 31:14850–14860
Ishizaki Y, Kira R, Fukuda M, Torisu H, Sakai Y, Sanefuji M, Yukaya N, Hara T (2009) Interleukin-10 is associated with resistance to febrile seizures: genetic association and experimental animal studies. Epilepsia 50:761–767
Zhou Z, Peng X, Insolera R, Fink DJ, Mata M (2009) Interleukin-10 provides direct trophic support to neurons. J Neurochem 110:1617–1627
McAuley JW, McFadden LS, Elliott JO, Shneker BF (2008) An evaluation of self management behaviours and medication adherence in patients with epilepsy. Epilepsy Behav 13:637–641
Perucca E, French J, Bialer M (2007) Development of new antiepileptic drugs: challenges, incentives, and recent advances. Lancet Neurol 6:793–804
Kaminski RM, Rogawski MA, Klitgaard H (2014) The potential of antiseizure drugs and agents that act on novel molecular targets as antiepileptogenic treatments. Neurotherapeutics 11:385–400
Kowski AB, Weissinger F, Gaus V, Fidzinski P, Losch F, Holtkamp M (2016) Specific adverse effects of antiepileptic drugs—A true-to-life monotherapy study. Epilepsy Behav 54:150–157
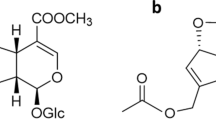
Jia N, Li Y et al (2016) Iridoid glycosides from the flowers of Gentiana macrophylla Pall. Ameliorate collagen-induced arthritis in rats. J Ethnopharmacol 189:1–9
Lu CM, Lin LC, Tsai TH (2014) Determination and pharmacokinetic study of gentiopicroside, geniposide, baicalin, and swertiamarin in chinese herbal formulae after oral administration in rats by LC-MS/MS. Molecules 19:21560–21578
Saravanan S, Pandikumar P et al (2014) In vivo and in vitro immunomodulatory potential of swertiamarin isolated from Enicostema axillare (Lam.) A. Raynal that acts as an anti-inflammatory agent. Inflammation 37:1374–1388
Saravanan S, Islam VI et al (2014) Swertiamarin attenuates inflammation mediators via modulating NF-κB/IκB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur J Pharm Sci 56:70–86
Jaishree V, Badami S, Rupesh Kumar M, Tamizhmani T (2009) Antinociceptive activity of swertiamarin isolated from enicostemma axillare. Phytomedicine 16(2–3):227–232
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Carrasco-Pozo C, Tan KN, Borges K (2015) Sulforaphane is anticonvulsant and improves mitochondrial function. J Neurochem 135:932–942
Brophy GM, Bell R et al (2012) Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 17:3–23
Iyengar SS, LaFrancois JJ et al (2015) Suppression of adult neurogenesis increases the acute effects of kainic acid. Exp Neurol 264:135–149
Liu G, Wang J, Deng XH, Yu JQ et al (2017) The anticonvulsant and neuroprotective effects of oxysophocarpine on pilocarpine-induced convulsions in adult male mice. Cell Mol Neurobiol 37(2):339–349
Anderson KJ, Miller KM, Fugaccia I, Scheff SW (2005) Regional distribution of Fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp Neurol 193:125–130
Kamphuis W, Mamber C et al (2012) GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS ONE 7:e42823
Duncan JS, Sander JW, Sisodiya SM, Walker MC (2006) Adult epilepsy. Lancet 367:1087–1100
Mazzuferi M, Kumar G, Rospo C, Kaminski RM (2012) Rapid epileptogenesis in the mouse pilocarpine model: video-EEG, pharmacokinetic and histopathological characterization. Exp Neurol 238:156–167
Xanthos DN, Sandkühler J (2014) Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 15:43–53
Dalkara S, Karakurt A (2012) Recent progress in anticonvulsant drug research: strategies for anticonvulsant drug development and applications of antiepileptic drugs for non-epileptic central nervous system disorders. Curr Top Med Chem 12:1033–1071
Henneberger C, Steinhäuser C (2016) Astrocytic TLR4 at the crossroads of inflammation and seizure susceptibility. J Cell Biol 215:607–609
Sloan SA, Barres BA (2014) Mechanisms of astrocyte development and their contributions to neurodevelopmental disorders. Curr Opin Neurobiol 27:75–81
Chung WS, Welsh CA, Barres BA, Stevens B (2015) Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci 18:1539–1545
Sherafat MA, Ronaghi A et al (2013) Kindling-induced learning deficiency and possible cellular and molecular involved mechanisms. Neurol Sci 34:883–890
Ortinski PI, Dong J et al (2010) Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13:584–591
Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36:174–184
Vezzani A, Baram TZ (2007) New roles for interleukin-1 beta in the mechanisms of epilepsy. Epilepsy Curr 7:45–50
Uludag IF, Duksal T, Tiftikcioglu BI, Zorlu Y, Ozkaya F, Kirkali G (2015) IL-1β, IL-6 and IL-1Ra levels in temporal lobe epilepsy. Seizure 26:22–25
Vezzani A, Aronica E, Mazarati A, Pittman QJ (2013) Epilepsy and brain inflammation. Exp Neurol 244:11–21
Xiao Z, Peng J, Yang L, Kong H, Yin F (2015) Interleukin-1β plays a role in the pathogenesis of mesial temporal lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in hippocampal neurons. J Neuroimmunol 282:110–117
Auvin S, Shin D, Mazarati A, Sankar R (2010) Inflammation induced by LPS enhances epileptogenesis in immature rat and may be partially reversed by IL1RA. Epilepsia 51:34–38
Ravizza T, Vezzani A (2006) Status epilepticus induces time-dependent neuronal and astrocytic expression of interleukin-1 receptor type I in the rat limbic system. Neuroscience 137:301–308
Wang Y, Qin ZH (2010) Molecular and cellular mechanisms of excitotoxic neuronal death. Apoptosis 15:1382–1402
Dey A, Kang X, Qiu J, Du Y, Jiang J (2016) Anti-inflammatory small molecules to treat seizures and epilepsy: from bench to bedside. Trends Pharmacol Sci 37:463–484
Rojas A, Jiang J, Ganesh T, Yang MS, Lelutiu N, Gueorguieva P, Dingledine R (2014) Cyclooxygenase-2 in epilepsy. Epilepsia 55:17–25
Sharma S, Yang B, Xi X, Grotta JC, Aronowski J, Savitz SI (2011) IL-10 directly protects cortical neurons by activating PI-3 kinase and STAT-3 pathways. Brain Res 1373:189–194
Alsharafi WA, Xiao B, Abuhamed MM, Bi F-F, Luo Z-H (2015) Correlation between IL-10 and microRNA-187 expression in epileptic rat hippocampus and patients with temporal lobe epilepsy. Front Cell Neurosci 9:466. doi:10.3389/fncel.2015.00466
Gouveia TL, Scorza FA et al (2014) Lovastatin decreases the synthesis of inflammatory mediators during epileptogenesis in the hippocampus of rats submitted to pilocarpine-induced epilepsy. Epilepsy Behav 36:68–73
Barker-Haliski ML, Heck TD, Dahle EJ, Vanegas F, Pruess TH, Wilcox KS, White HS (2016) Acute treatment with minocycline, but not valproic acid, improves long-term behavioral outcomes in the Theiler’s virus model of temporal lobe epilepsy. Epilepsia 57:1958–1967
Mishra A, Goel RK (2015) Comparative behavioral and neurochemical analysis of phenytoin and valproate treatment on epilepsy induced learning and memory deficit: search for add on therapy. Metab Brain Dis 30:951–958
Borham LE, Mahfoz AM et al (2016) The effect of some immunomodulatory and anti-inflammatory drugs on Li-pilocarpine-induced epileptic disorders in wistar rats. Brain Res 1648:418–424
Ezz HS, Khadrawy YA, Noor NA (2011) The neuroprotective effect of curcumin and nigella sativa oil against oxidative stress in the pilocarpine model of epilepsy: a comparison with valproate. Neurochem Res 36:2195–2204
Acknowledgements
This study was supported by the Ningxia Hui Autonomous Region science and technology support program (Grant No. 2015BAK45B01) and the Ningxia 13th Plan of 5-year major scientific program (Grant No. 2016BZ 07). We are indebted to the staff in the Animal Center and the Science and Technology Centre who provided assistance in the study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Deng, XH., Zhang, X., Wang, J. et al. Anticonvulsant Effect of Swertiamarin Against Pilocarpine-Induced Seizures in Adult Male Mice. Neurochem Res 42, 3103–3113 (2017). https://doi.org/10.1007/s11064-017-2347-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2347-0