Abstract
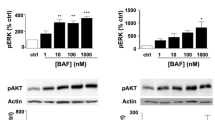
Multiple sclerosis (MS) is a demyelinating disorder characterized by massive neurodegeneration and profound axonal loss. Since myelin is enriched with sphingolipids and some of them display toxicity, biological function of sphingolipids in demyelination has been investigated in MS brain tissues. An elevation of sphingosine with a decrease in monoglycosylceramide and psychosine (myelin markers) was observed in MS white matter and plaque compared to normal brain tissue. This indicated that sphingosine toxicity might mediate oligodendrocyte degeneration. To explain the source of sphingosine accumulation, total sphingolipid profile was investigated in Lewis rats after inducing experimental autoimmune encephalomyelitis (EAE) and also in human oligodendrocytes in culture. An intermittent increase in ceramide followed by sphingosine accumulation in EAE spinal cord along with a stimulation of serine-palmitoyltransferase (SPT) activity was observed. Apoptosis was identified in the lumbar spinal cord, the most prominent demyelinating area, in the EAE rats. TNFα and IFNγ stimulation of oligodendrocytes in culture also led to an accumulation of ceramide with an elevation of sphingosine. Ceramide elevation was drastically blocked by myriocin, an inhibitor of SPT, and also by FTY720. Myriocin treatment also protected oligodendrocytes from cytokine mediated apoptosis or programmed cell death. Hence, we propose that sphingosine toxicity may contribute to demyelination in both EAE and MS, and the intermittent ceramide accumulation in EAE may, at least partly, be mediated via SPT activation, which is a novel observation that has not been previously reported.








Similar content being viewed by others
Abbreviations
- EAE:
-
Experimental autoimmune encephalomyelitis
- FMCs:
-
Fast migrating cerebrosides
- GalCer:
-
Galactosylceramide
- GC-MS:
-
Gas chromatography-mass spectrometry.
- HPLC:
-
High performance liquid chromatography
- HPTLC:
-
High performance thin-layer chromatography
- MGC:
-
Monoglycosylceramide
- MS:
-
Multiple sclerosis
- NAWM:
-
Normal appearing white matter
- SC:
-
Spinal cord
- SPT:
-
Serine palmitoyltransferase
References
Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278–285
Tsareva E, Kulakova O, Boyko A, Favorova O (2016) Pharmacogenetics of multiple sclerosis: personalized therapy with immunomodulatory drugs. Pharmacogenet Genom 26:103–115
Hunter SF (2016) Overview and diagnosis of multiple sclerosis. Am J Manag Care 22:s141–s150
Navikas V, Link H (1996) Review: cytokines and the pathogenesis of multiple sclerosis. J Neurosci Res 45:322–333
Martino G, Poliani PL, Furlan R et al (2000) Cytokine therapy in immune-mediated demyelinating diseases of the central nervous system: a novel gene therapy approach. J Neuroimmunol 107:184–190
Deckx N, Lee WP, Berneman ZN, Cools N (2013) Neuroendocrine immunoregulation in multiple sclerosis. Clin Dev Immunol 2013:705232
Genain CP, Cannella B, Hauser SL, Raine CS (1999) Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 5:170–175
Dasgupta S, Hogan EL (2001) Chromatographic resolution and quantitative assay of CNS tissue sphingoids and sphingolipids. J Lipid Res 42:301–308
Kieseier BC, Storch MK, Archelos JJ, Martino G, Hartung HP (1999) Effector pathways in immune mediated central nervous system demyelination. Curr Opin Neurol 12:323–336
McCoy MK, Tansey MG (2008) TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation 5:45
Jana A, Pahan K (2010) Sphingolipids in multiple sclerosis. Neuromolecular Med 12:351–361
Bhat NR, Zhang P (1996) Activation of mitogen-activated protein kinases in oligodendrocytes. J Neurochem 66:1986–1994
Andrews T, Zhang P, Bhat NR (1998) TNFalpha potentiates IFNgamma-induced cell death in oligodendrocyte progenitors. J Neurosci Res 54:574–583
Krown KA, Page MT, Nguyen C et al (1996) Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest 98:2854–2865
Eng LF, Ghirnikar RS, Lee YL (1996) Inflammation in EAE: role of chemokine/cytokine expression by resident and infiltrating cells. Neurochem Res 21:511–525
Kunz M, Ibrahim SM (2009) Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Med Inflamm 2009:979258
Castellano V, Patel DI, White LJ (2008) Cytokine responses to acute and chronic exercise in multiple sclerosis. J Appl Physiol 104:1697–1702
Haughey NJ (2010) Sphingolipids in neurodegeneration. Neuromolecular Med 12:301–305
Moscatelli EA, Isaacson E (1969) Gas liquid chromatographic analysis of sphingosine bases in sphingolipids of human normal and multiple sclerosis cerebral white matter. Lipids 4:550–555
Dasgupta S.S. et al (2000) Sphingolipid level in demyelinating disease. J. Neurochem 74:S34D
Qin J, Berdyshev E, Goya J, Natarajan V, Dawson G (2010) Neurons and oligodendrocytes recycle sphingosine 1-phosphate to ceramide: significance for apoptosis and multiple sclerosis. J Biol Chem 285:14134–14143
Henderson AP, Barnett MH, Parratt JD, Prineas JW (2009) Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol 66:739–753
Martin R, Sospedra M, Rosito M, Engelhardt B (2016) Current multiple sclerosis treatments have improved our understanding of MS autoimmune pathogenesis. Eur J Immunol 46:2078–2090
Buntinx M, Vanderlocht J, Hellings N et al (2003) Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression. J Neurocytol 32:25–38
Ray SK, Schaecher KE, Shields DC, Hogan EL, Banik NL (2000) Combined TUNEL and double immunofluorescent labeling for detection of apoptotic mononuclear phagocytes in autoimmune demyelinating disease. Brain Res Protoc 5:305–311
Igisu H, Suzuki K (1984) Analysis of galactosylsphingosine (psychosine) in the brain. J Lipid Res 25:1000–1006
Ray SK, Shields DC, Saido TC et al (1999) Calpain activity and translational expression increased in spinal cord injury. Brain Res 816:375–380
Williams RD, Wang E, Merrill AH Jr (1984) Enzymology of long-chain base synthesis by liver: characterization of serine palmitoyltransferase in rat liver microsomes. Arch Biochem Biophys 228:282–291
Perry DK, Carton J, Shah AK, Meredith F, Uhlinger DJ, Hannun YA (2000) Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J Biol Chem 275:9078–9084
Wang G, Krishnamurthy K, Chiang YW, Dasgupta S, Bieberich E (2008) Regulation of neural progenitor cell motility by ceramide and potential implications for mouse brain development. J Neurochem 106:718–733
Dasgupta S, Yanagisawa M, Krishnamurthy K, Liour SS, Yu RK (2007) Tumor necrosis factor-alpha up-regulates glucuronosyltransferase gene expression in human brain endothelial cells and promotes T-cell adhesion. J Neurosci Res 85:1086–1094
Krishnamurthy K, Dasgupta S, Bieberich E (2007) Development and characterization of a novel anti-ceramide antibody. J Lipid Res 48:968–975
Chun J, Hartung HP (2010) Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33:91–101
Lahiri S, Park H, Laviad EL, Lu X, Bittman R, Futerman AH (2009) Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and noncompetitive inhibition in an Acyl-CoA chain length-dependent manner. J Biol Chem 284:16090–16098
Berdyshev EV, Gorshkova I, Skobeleva A et al (2009) FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem 284:5467–5477
Dasgupta S, Everhart MB, Bhat NR, Hogan EL (1997) Neutral monoglycosylceramides in rat brain: occurrence, molecular expression and developmental variation. Dev Neurosci 19:152–161
Dasgupta S, Levery SB, Hogan EL (2002) 3-O-acetyl-sphingosine-series myelin glycolipids: characterization of novel 3-O-acetyl-sphingosine galactosylceramide. J Lipid Res 43:751–761
Hannun YA, Bell RM (1987) Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science 235:670–674
Phillips DC, Martin S, Doyle BT, Houghton JA (2007) Sphingosine-induced apoptosis in rhabdomyosarcoma cell lines is dependent on pre-mitochondrial Bax activation and post-mitochondrial caspases. Cancer Res 67:756–764
Plo I, Ghandour S, Feutz AC, Clanet M, Laurent G, Bettaieb A (1999) Involvement of de novo ceramide biosynthesis in lymphotoxin-induced oligodendrocyte death. Neuroreport 10:2373–2376
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150
Mathias S, Pena LA, Kolesnick RN (1998) Signal transduction of stress via ceramide. Biochem J 335(3):465–480
Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S (2003) The immunosuppressant FTY720 is phosphorylated by sphingosine kinase type 2. FEBS Lett 554:189–193
Hoffmann A, Grimm C, Kraft R et al (2010) TRPM3 is expressed in sphingosine-responsive myelinating oligodendrocytes. J Neurochem 114:654–665
Dasgupta S, Bhat NR, Spicer SS, Hogan EL, Furuya S, Hirabayashi Y (2007) Cell-specific expression of neutral glycosphingolipids in vertebrate brain: immunochemical localization of 3-O-acetyl-sphingosine-series glycolipid(s) in myelin and oligodendrocytes. J Neurosci Res 85:2856–2862
Singh I, Pahan K, Khan M, Singh AK (1998) Cytokine-mediated induction of ceramide production is redox-sensitive. Implications to proinflammatory cytokine-mediated apoptosis in demyelinating diseases. J Biol Chem 273:20354–20362
Miyata S, Hattori T, Shimizu S, Ito A, Tohyama M (2015) Disturbance of oligodendrocyte function plays a key role in the pathogenesis of schizophrenia and major depressive disorder. Biomed Res Int 2015:492367
Yu CS, Zhu CZ, Li KC et al (2007) Relapsing neuromyelitis optica and relapsing-remitting multiple sclerosis: differentiation at diffusion-tensor MR imaging of corpus callosum. Radiology 244:249–256
Filippi M, Campi A, Dousset V et al (1995) A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology 45:478–482
Filippi M (2001) Linking structural, metabolic and functional changes in multiple sclerosis. Eur J Neurol 8:291–297
Wheeler D, Bandaru VV, Calabresi PA, Nath A, Haughey NJ (2008) A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain 131:3092–3102
Schiffmann S, Ferreiros N, Birod K et al (2012) Ceramide synthase 6 plays a critical role in the development of experimental autoimmune encephalomyelitis. J Immunol 188:5723–5733
Giacoppo S, Soundara Rajan T, Galuppo M et al (2015) Purified Cannabidiol, the main non-psychotropic component of Cannabis sativa, alone, counteracts neuronal apoptosis in experimental multiple sclerosis. Eur Rev Med Pharmacol Sci 19:4906–4919
Zheng W, Kollmeyer J, Symolon H et al (2006) Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim Biophys Acta 1758:1864–1884
Mandon EC, van Echten G, Birk R, Schmidt RR, Sandhoff K (1991) Sphingolipid biosynthesis in cultured neurons. Down-regulation of serine palmitoyltransferase by sphingoid bases. Eur J Biochem 198:667–674
Dawkins JL, Hulme DJ, Brahmbhatt SB, Auer-Grumbach M, Nicholson GA (2001) Mutations in SPTLC1, encoding serine palmitoyltransferase, long chain base subunit-1, cause hereditary sensory neuropathy type I. Nat Genet 27:309–312
Bejaoui K, Wu C, Scheffler MD et al (2001) SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat Genet 27:261–262
Inui K, Nishimoto J, Taniike M et al (1990) Study of pathogenesis in twitcher mouse, an enzymatically authentic model of Krabbe’s disease. J Neurol Sci 100:124–130
Matsuda M, Tsukada N, Koh CS, Iwahashi T, Shimada K, Yanagisawa N (1994) Expression of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 in the spinal cord of rats during acute experimental allergic encephalomyelitis. Autoimmunity 19:15–22
Pender MP, Nguyen KB, McCombe PA, Kerr JF (1991) Apoptosis in the nervous system in experimental allergic encephalomyelitis. J Neurol Sci 104:81–87
Merrill AH Jr (2002) De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem 277:25843–25846
Meyer SG, de Groot H (2003) Cycloserine and threo-dihydrosphingosine inhibit TNF-alpha-induced cytotoxicity: evidence for the importance of de novo ceramide synthesis in TNF-alpha signaling. Biochim Biophys Acta 1643:1–4
Kanno T, Nishimoto T, Fujita Y, Gotoh A, Nakano T, Nishizaki T (2012) Sphingosine induces apoptosis in MKN-28 human gastric cancer cells in an SDK-dependent manner. Cell Physiol Biochem 30:987–994
Ullio C, Casas J, Brunk UT et al (2012) Sphingosine mediates TNFalpha-induced lysosomal membrane permeabilization and ensuing programmed cell death in hepatoma cells. J Lipid Res 53:1134–1143
Woodcock J (2006) Sphingosine and ceramide signalling in apoptosis. IUBMB Life 58:462–466
Kagedal K, Zhao M, Svensson I, Brunk UT (2001) Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem J 359:335–343
Acknowledgements
We thank late Ms. Elaine Terry and Ms. Denise Matzelle for excellent laboratory assistance. This work was supported in part by these grants: NINDS-NS-31355 (SD), NIAAA-11865 (SD), SC State Appropriation # CR22 (SD), NINDS-NS-38146 (NLB), NINDS-NS-41088 (NLB), the National Multiple Sclerosis Society RG-2130 (NLB), NCI-CA-91460 (SKR), NINDS-NS-057811 (SKR), and SC SCIRF-2015-I-0 (SKR). We acknowledge the encouragement and support from Dr. E. L. Hogan, Ex-chair, Department of Neurology, Medical University of South Carolina (MUSC), Charleston, SC, USA and also Dr. David Perry, Department of Biochemistry, MUSC, Charleston, SC, USA for his advice on SPT assay. We acknowledge Prof. E. Bieberich, Augusta University, Augusta, GA, USA for supplying essential reagents and critically reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lawrence G. Miller Jr. and Jennifer A. Young contributed equally and were recipients of Summer undergraduate fellowships.
Rights and permissions
About this article
Cite this article
Miller, L.G., Young, J.A., Ray, S.K. et al. Sphingosine Toxicity in EAE and MS: Evidence for Ceramide Generation via Serine-Palmitoyltransferase Activation. Neurochem Res 42, 2755–2768 (2017). https://doi.org/10.1007/s11064-017-2280-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2280-2