Abstract
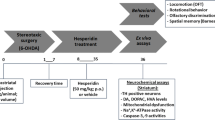
Parkinson’s disease (PD) is a neurodegenerative disorder due to loss of dopaminergic neurons in the substantia nigra pars compacta (SNC). PD finally leads to incapacitating symptoms including motor and cognitive deficits. This study was undertaken to assess protective effect of the flavanone hesperetin against striatal 6-hydroxydopamine lesion and to explore in more detail some underlying mechanisms including apoptosis, inflammation and oxidative stress. In this research study, intrastriatal 6-hydroxydopamine (6-OHDA)-lesioned rats received hesperetin (50 mg/kg/day) for 1 week. Hesperetin reduced apomorphine-induced rotational asymmetry and decreased the latency to initiate and the total time on the narrow beam task. It also attenuated striatal malondialdehyde and enhanced striatal catalase activity and GSH content, lowered striatal level of glial fibrillary acidic protein as an index of astrogliosis and increased Bcl2 with no significant change of the nuclear factor NF-kB as a marker of inflammation. Hesperetin treatment was also capable to mitigate nigral DNA fragmentation as an index of apoptosis and to prevent loss of SNC dopaminergic neurons. This study indicated the protective effect of hesperetin in an early model of PD via attenuation of apoptosis, astrogliosis marker and oxidative stress and it may be helpful as an adjuvant therapy for management of PD at its early stages.





Similar content being viewed by others
References
Massano J, Bhatia KP (2012) Clinical approach to Parkinson’s disease: features, diagnosis, and principles of management. Cold Spring Harb Perspect Med 2:a008870
Jannetta PJ, Whiting DM, Fletcher LH, Hobbs JK, Brillman J, Quigley M, Fukui M, Williams R (2011) Parkinson’s disease: an inquiry into the etiology and treatment. Neurol Int 3:e7
Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson’s disease. Mov Disord 26:1049–1055
Seidl SE, Santiago JA, Bilyk H, Potashkin JA (2014) The emerging role of nutrition in Parkinson’s disease. Front Aging Neurosci 6:36
Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I (2007) Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr 61:472–477
Palit S, Kar S, Sharma G, Das PK (2015) Hesperetin induces apoptosis in breast carcinoma by triggering accumulation of ROS and activation of ASK1/JNK pathway. J Cell Physiol 230:1729–1739
Wang J, Zhu H, Yang Z, Liu Z (2013) Antioxidative effects of hesperetin against lead acetate-induced oxidative stress in rats. Indian J Pharmacol 45:395–398
Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M (2015) Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res 29:323–331
Yang HL, Chen SC, Senthil Kumar KJ, Yu KN, Lee Chao PD, Tsai SY, Hou YC, Hseu YC (2012) Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: an ex vivo approach. J Agric Food Chem 60:522–532
Yang Z, Liu Y, Deng W, Dai J, Li F, Yuan Y, Wu Q, Zhou H, Bian Z, Tang Q (2014) Hesperetin attenuates mitochondria-dependent apoptosis in lipopolysaccharide-induced H9C2 cardiomyocytes. Mol Med Rep 9:1941–1946
Hwang SL, Lin JA, Shih PH, Yeh CT, Yen GC (2012) Pro-cellular survival and neuroprotection of citrus flavonoid: the actions of hesperetin in PC12 cells. Food Funct 3:1082–1090
Hwang SL, Shih PH, Yen GC (2012) Neuroprotective effects of citrus flavonoids. J Agric Food Chem 60:877–885
Antunes MS, Goes AT, Boeira SP, Prigol M, Jesse CR (2014) Protective effect of hesperidin in a model of Parkinson’s disease induced by 6-hydroxydopamine in aged mice. Nutrition 30:1415–1422
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
Choi EJ, Ahn WS (2008) Neuroprotective effects of chronic hesperetin administration in mice. Arch Pharm Res 31:1457–1462
Roghani M, Niknam A, Jalali-Nadoushan MR, Kiasalari Z, Khalili M, Baluchnejadmojarad T (2010) Oral pelargonidin exerts dose-dependent neuroprotection in 6-hydroxydopamine rat model of hemi-parkinsonism. Brain Res Bull 82:279–283
Allbutt HN, Henderson JM (2007) Use of the narrow beam test in the rat, 6-hydroxydopamine model of Parkinson’s disease. J Neurosci Methods 159:195–202
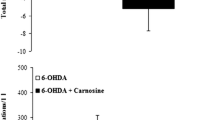
Afshin-Majd S, Khalili M, Roghani M, Mehranmehr N, Baluchnejadmojarad T (2015) Carnosine exerts neuroprotective effect against 6-hydroxydopamine toxicity in hemiparkinsonian rat. Mol Neurobiol 51:1064–1070
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC Handbook of methods for oxygen radical research. CRC, Boca Raton, pp 283–284
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Morroni F, Tarozzi A, Sita G, Bolondi C, Zolezzi Moraga JM, Cantelli-Forti G, Hrelia P (2013) Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson’s disease. Neurotoxicology 36:63–71
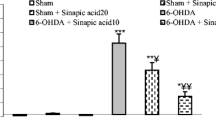
Zare K, Eidi A, Roghani M, Rohani AH (2015) The neuroprotective potential of sinapic acid in the 6-hydroxydopamine-induced hemi-parkinsonian rat. Metab Brain Dis 30:205–213
Healy-Stoffel M, Omar Ahmad S, Stanford JA, Levant B (2014) Differential effects of intrastriatal 6-hydroxydopamine on cell number and morphology in midbrain dopaminergic subregions of the rat. Brain Res 1574:113–119
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224
Schwarting RK, Huston JP (1997) Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology 18:689–708
Rainey-Smith S, Schroetke LW, Bahia P, Fahmi A, Skilton R, Spencer JP, Rice-Evans C, Rattray M, Williams RJ (2008) Neuroprotective effects of hesperetin in mouse primary neurones are independent of CREB activation. Neurosci Lett 438:29–33
Hwang SL, Yen GC (2011) Effect of hesperetin against oxidative stress via ER- and TrkA-mediated actions in PC12 cells. J Agric Food Chem 59:5779–5785
Jalali-Nadoushan M, Roghani M (2013) Alpha-lipoic acid protects against 6-hydroxydopamine-induced neurotoxicity in a rat model of hemi-parkinsonism. Brain Res 1505:68–74
Baranyi M, Milusheva E, Vizi ES, Sperlagh B (2006) Chromatographic analysis of dopamine metabolism in a Parkinsonian model. J Chromatogr A 1120:13–20
Und Halbach OVB, Schober A, Krieglstein K (2004) Genes, proteins, and neurotoxins involved in Parkinson’s disease. Prog Neurobiol 73:151–177
Chen S, Le W (2006) Neuroprotective therapy in Parkinson disease. Am J Ther 13:445–457
Kumar B, Gupta SK, Srinivasan BP, Nag TC, Srivastava S, Saxena R, Jha KA (2013) Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc Res 87:65–74
Zhou F, Wu JY, Sun XL, Yao HH, Ding JH, Hu G (2007) Iptakalim alleviates rotenone-induced degeneration of dopaminergic neurons through inhibiting microglia-mediated neuroinflammation. Neuropsychopharmacology 32:2570–2580
Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL (2006) Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol 197:275–283
Sriram K, O’Callaghan JP (2007) Divergent roles for tumor necrosis factor-alpha in the brain. J Neuroimmune Pharmacol 2:140–153
Hwang CK, Chun HS (2012) Isoliquiritigenin isolated from licorice Glycyrrhiza uralensis prevents 6-hydroxydopamine-induced apoptosis in dopaminergic neurons. Biosci Biotechnol Biochem 76:536–543
Deng W, Jiang D, Fang Y, Zhou H, Cheng Z, Lin Y, Zhang R, Zhang J, Pu P, Liu Y, Bian Z, Tang Q (2013) Hesperetin protects against cardiac remodelling induced by pressure overload in mice. J Mol Histol 44:575–585
Trivedi PP, Kushwaha S, Tripathi DN, Jena GB (2011) Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovasc Toxicol 11:215–225
Calou I, Bandeira MA, Aguiar-Galvao W, Cerqueira G, Siqueira R, Neves KR, Brito GA, Viana G (2014) Neuroprotective properties of a standardized extract from Myracrodruon urundeuva Fr. All. (Aroeira-Do-Sertao), as evaluated by a Parkinson’s disease model in rats. Parkinsons Dis 2014:519615
Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M (2014) Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—a mini-review. Life Sci 113:1–6
Cho J (2006) Antioxidant and neuroprotective effects of hesperidin and its aglycone hesperetin. Arch Pharm Res 29:699–706
Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT (2005) Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson’s disease. Free Radic Res 39:1119–1125
Bagheri M, Joghataei MT, Mohseni S, Roghani M (2011) Genistein ameliorates learning and memory deficits in amyloid beta(1–40) rat model of Alzheimer’s disease. Neurobiol Learn Mem 95:270–276
Yin SM, Zhao D, Yu DQ, Li SL, An D, Peng Y, Xu H, Sun YP, Wang DM, Zhao J, Zhang WQ (2014) Neuroprotection by scorpion venom heat resistant peptide in 6-hydroxydopamine rat model of early-stage Parkinson’s disease. Acta Physiol Sin 66:658–666
Park SE, Song KI, Suh JK, Hwang D, Youn I (2015) A time-course study of behavioral and electrophysiological characteristics in a mouse model of different stages of Parkinson’s disease using 6-hydroxydopamine. Behav Brain Res 284:153–157
Yuan H, Liang LW, Chen ZJ, Ji HR, Wang MK, Zhang HY, Li C, Xu JY (2006) R-apomorphine protects against 6-hydroxydopamine-induced nigrostriatal damage in rat. Neurosci Bull 22:331–338
Richter F, Hamann M, Richter A (2008) Moderate degeneration of nigral neurons after repeated but not after single intrastriatal injections of low doses of 6-hydroxydopamine in mice. Brain Res 1188:148–156
Roghani M, Behzadi G, Baluchnejadmojarad T (2002) Efficacy of elevated body swing test in the early model of Parkinson’s disease in rat. Physiol Behav 76:507–510
Acknowledgments
This research study was financially supported by a grant from Neurophysiology Research Center affiliated to Shahed University (Tehran, Iran) in 2013.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict interest
The authors hereby report that there is no declaration of interest and they are responsible for the content of the paper.
Rights and permissions
About this article
Cite this article
Kiasalari, Z., Khalili, M., Baluchnejadmojarad, T. et al. Protective Effect of Oral Hesperetin Against Unilateral Striatal 6-Hydroxydopamine Damage in the Rat. Neurochem Res 41, 1065–1072 (2016). https://doi.org/10.1007/s11064-015-1796-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1796-6