Abstract
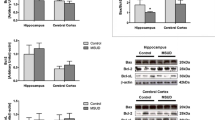
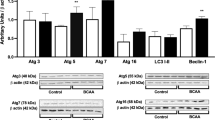
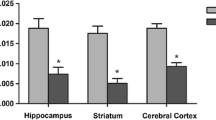
Maple syrup urine disease (MSUD) is caused by an inborn error in metabolism resulting from a deficiency in the branched-chain α-keto acid dehydrogenase complex activity. This blockage leads to accumulation of the branched-chain amino acids (BCAA) leucine, isoleucine and valine, as well as their corresponding α-keto acids and α-hydroxy acids. High levels of BCAAs are associated with neurological dysfunction and the role of pro- and mature brain-derived neurotrophic factor (BDNF) in the neurological dysfunction of MSUD is still unclear. Thus, in the present study we investigated the effect of an acute BCAA pool administration on BDNF levels and on the pro-BDNF cleavage-related proteins S100A10 and tissue plasminogen activator (tPA) in rat brains. Our results demonstrated that acute Hyper-BCAA (H-BCAA) exposure during the early postnatal period increases pro-BDNF and total-BDNF levels in the hippocampus and striatum. Moreover, tPA levels were significantly decreased, without modifications in the tPA transcript levels in the hippocampus and striatum. On the other hand, the S100A10 mRNA and S100A10 protein levels were not changed in the hippocampus and striatum. In the 30-day-old rats, we observed increased pro-BDNF, total-BDNF and tPA levels only in the striatum, whereas the tPA and S100A10 mRNA expression and the immunocontent of S100A10 were not altered. In conclusion, we demonstrated that acute H-BCAA administration increases the pro-BDNF/total-BDNF ratio and decreases the tPA levels in animals, suggesting that the BCAA effect may depend, at least in part, on changes in BDNF post-translational processing.






Similar content being viewed by others
References
Treacy E, Clow CL, Reade TR, Chitayat D, Mamer OA, Scriver CR (1992) Maple syrup urine disease: interrelationship between branched-chain amino-, oxo- and hydroxyacids; implications for treatment; associations with CNS dysmyelination. J Inherit Metab Dis 15:121–135
Chuang DT, Shih VE (2001) Maple syrup urine disease (branched chain ketoaciduria). In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1971–2005
Schönberger S, Schweiger B, Schwahn B, Schwarz M, Wendel U (2004) Dysmyelination in the brain of adolescents and young adults with maple syrup urine disease. Mol Genet Metab 82:69–75
Halestrap AP, Brand MD, Denton RM (1974) Inhibition of mitochondrial pyruvate transport by phenylpyruvate and -ketoisocaproate. Biochem Biophys Acta 367:102–108
Land JM, Mowbray J, Clark JB (1976) Control of pyruvate and h-hydroxybutyrate utilization in rat brain mitochondria and its relevance to phenylketonuria and maple syrup urine disease. J Neurochem 26:823–830
Danner DJ, Elsas LJ (1989) Disorders of branched chain amino acid and keto acid metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic basis of inherited disease. McGraw-Hill, New York, pp 671–692
Yudkoff M, Daikhin Y, Lin ZP, Nissim I, Stern J, Pleasure D, Nissim I (1994) Interrelationships of leucine and glutamate metabolism in cultured astrocyts. J Neurochem 62:1192–1202
Zielke HR, Zielke CL, Baab PJ, Collins RM (2002) Large neutral amino acids auto exchange when infused by microdialysis into the rat brain: implications for maple syrup urine disease and phenylketonuria. Neurochem Int 40:347–354
Pilla C, Cardozo RF, Dornelles PK, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2003) Kinetic studies on the inhibition of creatine kinase activity by branched-chain alpha-amino acids in the brain cortex of rats. Int J Dev Neurosci 21:145–151
Pilla C, Cardozo RF, Dutra-Filho CS, Wyse AT, Wajner M, Wannmacher CM (2003) Creatine kinase activity from rat brain is inhibited by branched chain amino acids in vitro. Neurochem Res 28:675–679
Prensky AL, Moser HW (1967) Changes in the amino acid composition of proteolipids of white matter during maturation of the human nervous system. J Neurochem 14:117–121
Dodd PR, Williams SH, Gundlach AL, Harper PA, Healy PJ, Dennis JA, Johnston GA (1992) Glutamate and aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J Neurochem 59:582–590
Tavares RG, Santos CES, Tasca C, Wajner M, Souza DO, Dutra-Filho CS (2000) Inhibition of glutamate uptake into synaptic vesicles of rat brain by the metabolites accumulating in maple syrup urine disease. J Neurol Sci 181:44–49
Araújo P, Wassermann GF, Tallini K, Furlanetto V, Vargas CR, Wannmacher CM, Dutra-Filho CS, Wyse AT, Wajner M (2001) Reduction of large neutral amino acid levels in plasma and brain of hyperleucinemic rats. Neurochem Int 38:529–537
Jouvet J, Rustin P, Taylor DL, Pocock JM, Felderhoff-Mueser U, Mazarakis ND, Sarraf C, Joashi U, Kozma M, Greenwood K, Edwards AD, Mehmet H (2000) Branched chain amino acids induce apoptosis in neural cells without mitochondrial membrane depolarization or cytochrome c release: implications for neurological impairment associated with maple syrup urine disease. Mol Biol Cell 11:1919–1932
Fontella FU, Gassen E, Pulrolnik V, Wannmacher CM, Klein AB, Wajner M, Dutra-Filho CS (2002) Stimulation of lipid peroxidation in vitro in rat brain by the metabolites accumulating in maple syrup urine disease. Metab Brain Dis 17:47–54
Bridi R, Araldi J, Sgarbi MB, Testa CG, Durigon K, Wajner M, Dutra-Filho CS (2003) Induction of oxidative stress in rat brain by the metabolites accumulating in maple syrup urine disease. Int J Dev Neurosci 21:327–332
Bridi R, Latini A, Braum CA, Zorzi GK, Moacir W, Lissi E, Dutra-Filho CS (2005) Evaluation of the mechanisms involved in leucine induced oxidative damage in cerebral cortex of young rats. Free Radic Res 39:71–79
Barschak AG, Sitta A, Deon M, de Oliveira MH, Haeser A, Dutra-Filho CS, Wajner M, Vargas CR (2006) Evidence that oxidative stress in increased in plasma from patients with maple syrup urine disease. Metab Brain Dis 21:279–286
Mescka C, Moraes T, Rosa A, Mazzola P, Piccoli B, Jacques C, Dalazen G, Coelho J, Cortes M, Terra M, Regla Vargas C, Dutra-Filho CS (2011) In vivo neuroprotective effect of L-carnitine against oxidative stress in maple syrup urine disease. Metab Brain Dis 26:21–28
Clarke PG (1985) Neuronal death during development in the isthmo-optic nucleus of the chick: sustaining role of afferents from the tectum. J Comp Neurol 234:365–379
Oppenheim RW (1991) Cell death during development of the nervous system. Annu Rev Neurosci 14:453–501
Vicario-Abejon C, Collin C, McKay RD, Segal M (1998) Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci 18:7256–7271
Luikart BW, Nef S, Virmani T, Lush ME, Liu Y, Kavalali ET, Parada LF (2005) TrkB has a cell-autonomous role in the establishment of hippocampal Schaffer collateral synapses. J Neurosci 25:3774–3786
Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24:677
Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72:609–642
Poo MM (2001) Neurotrophins as synaptic modulators. Nat Rev Neurosci 2:24–32
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299–309
Lu B, Je HS (2003) Neurotrophic regulation of the development and function of the neuromuscular synapses. J Neurocytol 32:931–941
Bramham CR (2008) Local protein synthesis, actin dynamics and LTP consolidation. Curr. Opin Neuorbiol 18:524–531
McAllister AK, Katz LC, Lo DC (1999) Neurotrophins and synaptic plasticity. Annu Rev Neurosci 22:295–318
Carvalho AL, Caldeira MV, Santos SD, Duarte CB (2008) Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br J Pharmacol 153:S310–S324
Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P (2001) BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature 411:86–89
Mossner R, Daniel S, Albert D, Heils A, Okladnova O, Schmitt A, Lesch KP (2000) Serotonin transporter function is modulated by brain-derived neurotrophic factor (BDNF) but not nerve growth factor (NGF). Neurochem Int 36:197–202
Gezen-Ak D, Dursun E, Hanagasi H, Bilgic B, Lohman E, Araz OS, Atasoy IL, Alaylıoğlu M, Önal B, Gürvit H, Yılmazer S (2013) BDNF, TNFalpha, HSP90, CFH, and IL-10 serum levels in patients with early or late onset alzheimer’s disease or mild cognitive impairment. J Alzheimers Dis 37:185–195
Peng S, Wuu J, Mufson EJ, Fahnestock M (2005) Precursor form of brain-derived neurotrophic factor and mature brainderived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J Neurochem 93:1412–1421
Yu H, Zhang Z, Shi Y, Bai F, Xie C, Qian Y, Yuan Y, Deng L (2008) Association study of the decreased serum BDNF concentrations in amnestic mild cognitive impairment and the Val66Met polymorphism in Chinese Han. J Clin Psychiatry 69:1104–111110
Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R (2005) Neurotrophin levels in postmortem brains of suicide victims and the effects of ante mortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 136:29–37
Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, Nakazato M, Watanabe H, Shinoda N, Okada S, Iyo M (2003) Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry 54:70–75
Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Goncalves CA, Santin A, Kapczinski F (2006) Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett 398:215–219
Terracciano A, Lobina M, Piras MG, Mulas A, Cannas A, Meirelles O, Sutin AR, Zonderman AB, Uda M, Crisponi L, Schlessinger D (2011) Neuroticism, depressive symptoms, and serum BDNF. Psychosom Med 73:638–642
Lessmann V, Gottmann K, Malcangio M (2003) Neurotrophin secretion: current facts and future prospects. Prog Neurobiol 69:341–374
Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T (1995) Hippocampal long-term potentiation is impaired in mice lacking brainderived neurotrophic factor. Proc Natl Acad Sci USA 92:8856–8860
Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B (1996) Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381:706–709
Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16:1137–1145
Lu B, Pang PT, Woo NH (2005) The yin and yang of neurotrophin action. Nat Rev Neurosci 6:603–614
Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294:1945–1948
Pang PT, Teng HK, Zaitsev E (2004) Cleavage of pro-BDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science 306:487–491
Kassam G, Le BH, Choi KS, Kang HM, Fitzpatrick SL, Louie P, Waisman DM (1998) The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry 37:16958–16966
Zobiack N, Rescher U, Ludwig C, Zeuschner D, Gerke V (2003) The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol Biol Cell 14:4896–4908
Bramham CR, Messaoudi E (2005) BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol 76(2):99–125
Goldberg TE, Weinberger DR (2004) Genes and the parsing of cognitive processes. Trends Cogn Sci 8:325–335
Scaini G, Teodorak BP, Jeremias IC, Morais MO, Mina F, Dominguini D, Pescador B, Comim CM, Schuck PF, Ferreira GC, Quevedo J, Streck EL (2012) Antioxidant administration prevents memory impairment in an animal model of maple syrup urine disease. Behav Brain Res 231:92–96
Scaini G, Comim CM, Oliveira GM, Pasquali MA, Quevedo J, Gelain DP, Moreira JC, Schuck PF, Ferreira GC, Bogo MR, Streck EL (2013) Chronic administration of branched-chain amino acids impairs spatial memory and increases brain-derived neurotrophic factor in a rat model. J Inherit Metab Dis 36:721–730
Glaser V, Carlini VP, Gabach L, Ghersi M, de Barioglio SR, Ramirez OA, Perez MF, Latini A (2010) The intra-hippocampal leucine administration impairs memory consolidation and LTP generation in rats. Cell Mol Neurobiol 30:1067–1075
VdeC Vasques, Brinco F, Wajner M (2005) Intrahippocampal administration of the branched-chain alpha-hydroxy acids accumulating in maple syrup urine disease compromises rat performance in aversive and non-aversive behavioral tasks. J Neurol Sci 232:11–21
Mello CF, Feksa L, Brusque AM, Wannmacher CM, Wajner M (1999) Chronic early leucine administration induces behavioral deficits in rats. Life Sci 65:747–755
Bridi R, Fontella FU, Pulrolnik V, Braun CA, Zorzi GK, Coelho D, Wajner M, Vargas CR, Dutra-Filho CS (2006) A chemically-induced acute model of maple syrup urine disease in rats for neurochemical studies. J Neurosci Methods 155:224–230
Lowry OH, Rosebough NG, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Chem Biol 193:265–275
Bonefeld BE, Elfving B, Wegener G (2008) Reference genes for normalization: a study of rat brain tissue. Synapse 62:302–309
Dimova EY, Samoylenko A, Kietzmann T (2004) Oxidative stress and hypoxia: implications for plasminogen activator inhibitor-1 expression. Antioxid Redox Signal 6:777–791
Zagotta I, Dimova EY, Funcke JB, Wabitsch M, Kietzmann T, Fischer-Posovszky P (2013) Resveratrol suppresses PAI-1 gene expression in a human in vitro model of inflamed adipose tissue. Oxid Med Cell Longev 2013:793525
Zervos IA, Nikolaidis E, Lavrentiadou SN, Tsantarliotou MP, Eleftheriadou EK, Papapanagiotou EP, Fletouris DJ, Georgiadis M, Taitzoglou IA (2011) Endosulfan-induced lipid peroxidation in rat brain and its effect on t-PA and PAI-1: ameliorating effect of vitamins C and E. J Toxicol Sci 36:423–433
Yamada K, Mizuno M, Nabeshima T (2000) Role for brain-derived neurotrophic factor in learning and memory. Life Sci 70:735–744
Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B (2005) Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci 8:1069–1077
Bekinschtein P, Cammarota M, Izquierdo I, Medina JH (2007) BDNF and memory formation and storage. Neuroscientist 14:147–156
Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I (2008) BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci USA 105:2711–2716
Lu Y, Christian K, Lu B (2008) BDNF: A key regulator for protein synthesis-dependent LTP and longterm memory? Neurobiol Learn Mem 89:312–323
Chao MV, Bothwell M (2002) Neurotrophins: to cleave or not to cleave. Neuron 33:9–12
Ibanez CF (2002) Jekyll-Hyde neurotrophins: the story of proNGF. Trends Neurosci 25:284–286
Bibel M, Barde YA (2000) Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev 14:2919–2937
Dechant G, Barde YA (2002) The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci 5:1131–1136
Schinder AF, Poo M (2000) The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci 23:639–645
Carlino D, Leone E, Di Cola F, Baj G, Marin R, Dinelli G, Tongiorgi E, De Vanna M (2011) Low serum truncated-BDNF isoform correlates with higher cognitive impairment in schizophrenia. J Psychiatr Res 45:273–279
Barnes P, Thomas KL (2008) Proteolysis of proBDNF is a key regulator in the formation of memory. PLoS One 3:e3248
Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA (2001) Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J Biol Chem 276:12660–12666
Acknowledgments
This research was supported by Grants from the Programa de Pós-graduação em Ciências da Saúde—Universidade do Extremo Sul Catarinense (UNESC), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Núcleo de Excelência em Neurociências Aplicadas de Santa Catarina (NENASC) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scaini, G., Morais, M.O.S., Furlanetto, C.B. et al. Acute Administration of Branched-Chain Amino Acids Increases the Pro-BDNF/Total-BDNF Ratio in the Rat Brain. Neurochem Res 40, 885–893 (2015). https://doi.org/10.1007/s11064-015-1541-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-015-1541-1