Abstract
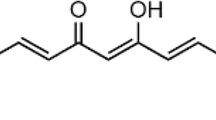
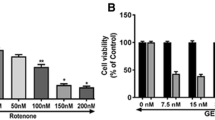
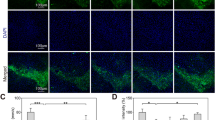
In the present study, using a human neuroblastoma SK-N-SH cells, we explored antioxidant, mitochondrial protective and antiapoptotic properties of mangiferin against rotenone-mediated cytotoxicity. SK-N-SH cells are divided into four experimental groups based on 3-(4,5-dimethyl2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay—untreated cells, cells incubated with rotenone (100 nM), cells treated with mangiferin (20 μg) (pretreatment 4 h before) + rotenone (100 nM) and mangiferin alone treated. 24 h after treatment with rotenone and 28 h after treatment with mangiferin, levels of ATP thiobarbituricacid reactive substances and reduced glutathione and activities of enzymatic antioxidants including superoxide dismutase, catalase and glutathione peroxidise were measured. Finally mitochondrial transmembrane potential and expressions of apoptotic protein were also analysed. Pre-treatment with mangiferin significantly enhanced cell viability, ameliorated decrease in mitochondrial membrane potential and decreased rotenone-induced apoptosis in the cellular model of Parkinson’s disease. Moreover oxidative imbalance induced by rotenone was partially rectified by mangiferin. Our results indicated that anti-apoptotic properties of this natural compound due to its antioxidant and mitochondrial protective function protect rotenone induced cytotoxicity.





Similar content being viewed by others
References
Shimizu K, Matsubara K, Ohtaki K, Shiono H, Shimizu K, Matsubara K, Ohtaki K et al (2003) Paraquat leads to dopaminergic neural vulnerability in organotypic midbrain culture. Neurosci Res 46:523–532
Kim HJ, Park HJ, Park HK, Chung JH (2009) Tranexamic acid protects against rotenone induced apoptosis in human neuroblastoma SH-SY5Y cells. Toxicology 262:171–174
Muthaiyah B, Essa MM, Chauhan V, Chauhan A (2011) Protective effects of walnut extract against amyloid beta peptide-induced cell death and oxidative stress in PC12 cells. Neurochem Res 36(11):2096–2103
Essa MM, Vijayan RK, Castellano-Gonzalez G, Memon MA, Braidy N, Guillemin GJ (2012) Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem Res 37(9):1829–1842
Braidy N, Selvaraju S, Essa MM, Vaishnav R, Al-Adawi S, Al-Asmi A, Al-Senawi H, Abd Alrahman Alobaidy A, Lakhtakia R, Guillemin GJ (2013) Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid Med Cell Longev 2013:685909. doi: 10.1155/2013/685909
Tamilselvam K, Braidy N, Manivasagam T, Essa MM, Prasad NR, Karthikeyan S, Thenmozhi AJ, Selvaraju S, Guillemin GJ (2013) Neuroprotective effects of hesperidin, a plant flavanone, on rotenone-induced oxidative stress and apoptosis in a cellular model for Parkinson’s disease. Oxid Med Cell Longev 2013:102741. doi:10.1155/2013/102741
Sánchez GM, Re L, Giuliani A, Núñez-Sellés AJ, Davison GP, León-Fernández OS et al (2000) Protective effects of Mangifera indica L. extract, mangiferin and selected antioxidants against TPA-induced biomolecules oxidation and peritoneal macrophage activation in mice. Pharmacol Res 42:565–573
Lemus-Molina Y, Sánchez-Gómez MV, Delgado-Hernández R, Matute C (2009) Mangifera indica L. extract attenuates glutamate-induced neurotoxicity on rat cortical neurons. Neurotoxicology 30:1053–1058
Gottlieb M, Leal-Campanario R, Campos-Esparza MR, Sanchez-Gomez MV, Alberdi E, Arranz A et al (2006) Neuroprotection by two polyphenols following excitotoxicity and experimental ischemia. Neurobiol Dis 2:374–386
Karthikeyan S, Kanimozhi G, Prasad NR, Mahalakshmi R (2011) Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol In Vitro 25:1366–1375
Niehaus WG Jr, Samuelsson B (1968) Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem 6(1):126–130
Del Maestro RF, McDonald W (1985) Oxidative enzymes in tissue homogenates. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 291–296
Aebi HE (1983) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinhern, pp 273–286
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Liberatore GT, Jackson-Lewis V, Vukosavic S et al (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5:1403–1409
Levites Y, Amit T, Mandel S, Youdim MB (2003) Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (−)-epigallocatechin-3-gallate. FASEB J. 17:952–954
Chung W, Miranda CL, Maier CS (2007) Epigallocatechin gallate (EGCG) potentiates the cytotoxicity of rotenone in neuroblastoma SH-SY5Y cells. Brain Res 1176:133–142
Turrens JF (1997) Superoxide production by the mitochondrial respiratory chain. Biosci Rep 17:3–8
Sherer T, Betarbetm R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT (2002) An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci 22:7006–7015
Cassarino DS, Fall CP, Swerdlow RH, Smith TS, Halvorsen EM, Millar SW, Parks JP, Parker WD, Bennett JP (1997) Elevated reactive oxygen species and antioxidant enzyme activities in animal and cellular models of Parkinson’s disease. Biochim Biophys Acta 1362:77–86
Kurosaki R, Muramatsu Y, Kato H, Araki T (2004) Biochemical, behavioral and immunohistochemical alterations in MPTP-treated mouse model of Parkinson’s disease. Pharmacol Biochem Behav 78:143–153
Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT et al (2005) Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem 280:42026–42035
Tatton WG, Olanow CW (1999) Apoptosis in neurodegenerative diseases: the role of mitochondria. Biochim Biophys Acta 1410:195–213
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J et al (2000) Caspase-12 mediates endoplasmic reticulum specific apoptosis and cytotoxicity by amyloid-β. Nature 403:98–103
Kweon GR, Marks JD, Krencik R, Leung EH, Schumacker PT, Hyland K et al (2004) Distinct mechanisms of neurodegeneration induced by chronic complex I inhibition in dopaminergic and non-dopaminergic cells. J Biol Chem 279:51783–51792
Hartley A, Stone JM, Heron C, Cooper JM, Schapira AHV (1994) Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson’s disease. J Neurochemistry 63(5):1987–1990
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kavitha, M., Manivasagam, T., Essa, M.M. et al. Mangiferin Antagonizes Rotenone: Induced Apoptosis Through Attenuating Mitochondrial Dysfunction and Oxidative Stress in SK-N-SH Neuroblastoma Cells. Neurochem Res 39, 668–676 (2014). https://doi.org/10.1007/s11064-014-1249-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1249-7