Abstract
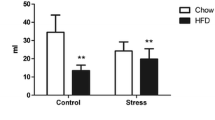
Social isolation is one of the most potent stressors in the prepubertal period and may influence disease susceptibility or resilience in adulthood. The glucocorticoid response and, consequently, the adaptive response to stress involve important changes in mitochondrial functions and apoptotic signaling. Previous studies have shown that consumption of a palatable diet reduces some stress effects. Therefore, the aim of the present study was to investigate whether isolation stress in early life can lead to cellular alterations in the hippocampus. For this, we evaluated oxidative stress parameters, DNA breakage index, mitochondrial mass and potential, respiratory chain enzyme activities, apoptosis, and necrosis in the hippocampus of juvenile male rats submitted or not to isolation stress during the pre-puberty period. We also verified whether consumption of a palatable diet during this period can modify stress effects. Results show that stress led to an oxidative imbalance, DNA breaks, increased the mitochondrial potential and early apoptosis, and decreased the number of live and necrotic cells. In addition, the palatable diet increased glutathione peroxidase activity, high mitochondrial potential and complex I–III activity in the hippocampus of juvenile rats. The administration of a palatable diet during the isolation period prevented the stress effects that caused the reduction in live cells and increased apoptosis. In conclusion, the stress experienced during the pre-pubertal period induced a hippocampal oxidative imbalance, DNA damage, mitochondrial dysfunction, and increased apoptosis, while consumption of a palatable diet attenuated some of these effects of exposure, such as the reduction in live cells and increased apoptosis, besides favoring an increase in antioxidant enzymes activities.





Similar content being viewed by others
References
McCormick CM, Mathews IZ (2007) HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav 86:220–233
Paus T, Keshavan M, Giedd JN (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957
Arakawa H (2005) Interaction between isolation rearing and social development on exploratory behavior in male rats. Behav Process 70:223–234
Ferdman N, Murmu RP, Bock J, Leshem M (2007) Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic andspine morphology in prefrontal cortex of rats. Behav Brain Res 180:174–182
Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J (2004) Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res 152:279–295
McEwen BS (2008) Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583:174–185
Sapolsky RM (2003) Stress and plasticity in the limbic system. Neurochem Res 28:1735–1742
McIntosh LJ, Sapolsky RM (1996) Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induce toxicity in neuronal culture. Exp Neurol 141:201–206
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Halliwell B, Gutteridge JM (2000) Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci 899:136–147
Kovacheva-Ivanova S, Bakalova R, Ribavov SR (1994) Immobilization stress enhances lipid’ peroxidation in the rat lungs. Materials and methods. Gen Physiol Biophys 13:469–482
Mitra R, Vyas A, Chatterjee G, Chattarji S (2005) Chronic stress induced modulation of different states of anxiety like behaviour in female rats. Neurosci Lett 383:278–283
Oishi K, Yokoi M, Maekawa S, Sodeyama C, Shiraishi T, Kondo R, Kuriyama T, Machida K (1999) Oxidative stress and haematological changes in immobilized rats. Acta Physiol Scand 165:65–69
Radak Z, Sasvari M, Nyakas C, Kaneko T, Tahara S, Ohno H, Goto S (2001) Single bout of exercise eliminates the immobilization-induced oxidative stress in rat brain. Neurochem Int 39:33–38
Krolow R, Noschang C, Weis SN, Pettenuzzo LF, Huffell AP, Arcego DM, Marcolin M, Mota CS, Kolling J, Scherer EB, Wyse AT, Dalmaz C (2012) Isolation stress during the prepubertal period in rats induces long-lasting neurochemical changes in the prefrontal cortex. Neurochem Res 37:63–73
Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, Chrousos GP (2007) Mitochondria as key components of the stress response. Trends Endocrinol Metab 18:190–198
McKernan DP, Dinan TG, Cryan JF (2009) “Killing the Blues”: a role for cellular suicide (apoptosis) in depression and the antidepressant response? Prog Neurobiol 88:246–263
Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2:647–656
Lindsten T, Zong WX, Thompson CB (2005) De fining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist 11:10–15
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489
Epel E, Lapidus R, McEwen B, Brownell K (2001) Stress may add bite to appetite in women. A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology 26:37–49
Adam TC, Epel ES (2007) Stress, eating and the reward system. Physiol Behav 91:449–458
Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF (2004) Chronic stress promotes palatable feeding, which reduces signs of stress. Feedforward and feed-back effects of chronic stress. Endocrinology 145:3754–3762
Higashimoto M, Isoyama N, Ishibashi S, Inoue M, Takiguchi M, Suzuki S, Ohnishi Y, Sato M (2009) Tissue-dependent preventive effect of metallothionein against DNA damage in dyslipidemic mice under repeated stresses of fasting or restraint. Life Sci 84:569–575
Muqbil I, Azmi AS, Banu N (2006) Prior exposure to restraint stress enhances 7,12-dimethylbenz(a)anthracene (DMBA) induced DNA damage in rats. FEBS Lett 580:3995–3999
Olivo-Marston SE, Zhu Y, Lee RY, Cabanes A, Khan G, Zwart A, Wang Y, Clarke R, Hilakivi-Clarke L (2008) Gene signaling pathways mediating the opposite effects of prepubertal low-fat and high-fat n 3 polyunsaturated fatty acid diets on mammary cancer risk. Cancer Prev Res 7:532–545
Douglas L, Varlinskaya E, Spear L (2004) Rewarding properties of social interactions in adolescent and adult male and female rates: impact of social versus isolate housing of subjects and partners. Dev Psychobiol 45:153–162
Delmas-Beauvieux MC, Peuchant E, Dumon MF, Receveur A, Le Bras M, Clerc M (1995) Relationship between red blood cell antioxidant enzymatic system status and lipoperoxidation during the acute phase of malaria. Clin Biochem 28:63–169
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Sriram K, Pai KS, Boyd MR, Ravindranath V (1997) Evidence for generation of oxidative stress in brain by MPTP: in vitro and in vivo studies in mice. Brain Res 21:44–52
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Rye JC, Sasaki YF (2000) Single cell gel/comet assay. Guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Collins A, Dusinská M, Franklin M, Somoroviská M, Petrovská H, Duthies S, Fillion LP, Panayiotidis M, Raslová K, Vaughan N (1997) Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ Mol Mutagen 30:139–146
Pendergrass W, Wolf N, Poot M (2004) Efficacy of MitoTracker Green and CMX rosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytometry 61:162–169
Khanal G, Chung K, Solis-Wever X, Johnsos B, Pappas O (2001) Ischemia/reperfusion injury of primary porcine cardiomyocytes in a low-shear microfluidic culture and analysis device. Analyst 36:19–26
Rodriguez-Enriquez S, Kai Y, Maldonado E, Currin RT, Lemasters JJ (2009) Roles of mitophagy and the mitochondrial permeability transition in remodeling of cultured rat hepatocytes. Autophagy 5:1099–1106
Weis SN, Pettenuzzo LF, Krolow R, Valentim LM, Mota CS, Dalmaz C (2012) Neonatal hypoxia-ischemia induces sex-related changes in rat brain mitochondria. Mitochondrion 12:271–279
Fischer JC, Ruitenbeek W, Berden JA, Trijbels JM, Veerkamp JH, Stadhouders AM, Sengers RC, Janssen AJ (1985) Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin Chim Acta 153:23–36
Rustin P, Chretien D, Bourgeron T, Gérard B, Rötig A, Saudubray JM, Minnich A (1994) Biochemical and molecular investigations in respiratory chain deficiencies. Clin Chim Acta 28:35–51
Schapira AH, Mann VM, Cooper JM (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55:2142–2145
Boersma AW, Nooter K, Oostrum RG, Stoter G (1996) Quantification of apoptotic cells with fluorescein isothiocyanate-labeled annexin V in chinese hamster ovary cell cultures treated with cisplatin. Cytometry 24:123–130
Homburg CH, de Haas M, von dem Borne AE, Verhoeven AJ, Reutelingsperger CP, Roos D (1995) Human neutrophils lose their surface Fc gamma RIII and acquire Annexin V binding sites during apoptosis in vitro. Blood 85:532–540
Martin SJ, Reutelingsperger CP, Mcgahon AJ, Rader JA, van Schie RC, La Face DM, Green DR (1995) Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182:1545–1556
Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C (1995) A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods 184:39–51
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Halliwell B, Gutteridge JMC (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford
Metodiewa D, Kóska C (2000) Reactive oxygen species and reactive nitrogen species: relevance to cyto(neuro)toxic events and neurologic disorders. An overview. Neurotox Res 1:197–233
Olanow CW (1992) An introduction to the free radical hypothesis in Parkinson’s disease. Ann Neurol 32:S2–S9
Holley AK, Dhar SK, Xu Y, St. Clair DK (2010) Manganese superoxide dismutase: beyond life and death. Amino Acids 42:139–158
Chakraborti A, Gulati K, Ray A (2008) Age related differences in stress-induced neurobehavioral responses in rats. Modulation by antioxidants and nitrergic agents. Behav Brain Res 194:86–91
Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, Leza JC (2001) Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology 24:420–429
Ward PA, Till GO (1990) Pathophysiologic events related to thermal injury of skin. J Trauma 30:S75–S79
El-Khamisy SF, Caldecott KW (2006) TDP1-dependent DNA single-strand break repair and neurodegeneration. Mutagenesis 21:219–224
Liu B, Chen Y, St. Clair DK (2008) ROS and p53: a versatile partnership. Free Radic Biol Med 44:1529–1535
Neuzil J, Wang XF, Dong LF, Low P, Ralph SJ (2006) Molecular mechanism of ‘mitocan’-induced apoptosis in cancer cells epitomizes the multiple roles of reactive oxygen species and Bcl-2 family proteins. FEBS Lett 580:5125–5129
Baisch H, Bollmann H, Bornkessel S (1999) Degradation of apoptic cells and fragments in HL60 suspension cultures after induction of apoptosis by camptothecin and ethanol. Cell Prolif 32:303–319
Brendler-Schwaab S, Hartmann A, Pfuhler S, Speit G (2005) The in vivo comet assay: use and status in genotoxicity testing. Mutagenesis 20:245–254
Krolow R, Noschang CG, Arcego D, Andreazza AC, Peres W, Gonçalves CA, Dalmaz C (2010) Consumption of a palatable diet by chronically stressed rats prevents effects on anxiety-like behavior but increases oxidative stress in a sex-specific manner. Appetite 55:108–116
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. Biochem J 134:707–716
Andreyev AY, Kushnareva YE, Starkov AA (2005) Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70:200–224
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40
Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, Okami N, Chan PH (2010) Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta 1802:92–99
Franklin JL (2011) Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid Redox Signal 14:1437–1448
Mancini M, Sedghinasab M, Knowlton K, Tam A, Hockenbery D, Anderson BO (1998) Flow cytometric measurement of mitochondrial mass and function: a novel method for assessing chemoresistance. Ann Surg Oncol 5:287–295
Mahyar-Roemer M, Katsen A, Mestres P, Roemer K (2001) Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int J Cancer 94:615–622
Kluza J, Marchetti P, Gallego MA, Lancel S, Fournier C, Loyens A, Beauvillain JC, Bailly C (2004) Mitochondrial proliferation during apoptosis induced by anticancer agents: effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene 23:7018–7030
Scarlett JL, Sheard PW, Hughes G, Ledgerwood EC, Ku HH, Murphy MP (2000) Changes in mitochondrial membrane potential during staurosporine induced apoptosis in Jurkat cells. FEBS Lett 475:267–272
Gottlieb E, Vander Heiden MG, Thompson CB (2000) Bcl-xL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol 20:5680–5689
Ortega-Camarillo C, Guzman-Grenfell AM, García-Macedo R, Rosales-Torres AM, Avalos-Rodrıguez A, Duran-Reyes G, Medina-Navarro G, Cruz M, Dıaz-Flores M, Kumate J (2006) Hyperglycemia induces apoptosis and p53 mobilization to mitochondria in RINm5F cells. Mol Cell Biochem 281:163–171
Acknowledgments
Financial support: National Research Council of Brazil (CNPq), and PRONEX-FAPERGS/CNPq 10/0018.3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krolow, R., Noschang, C., Arcego, D.M. et al. Isolation Stress Exposure and Consumption of Palatable Diet During the Prepubertal Period Leads to Cellular Changes in the Hippocampus. Neurochem Res 38, 262–272 (2013). https://doi.org/10.1007/s11064-012-0915-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0915-x