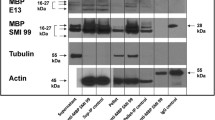
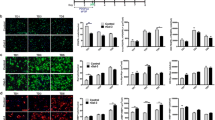
Abstract
The 18.5-kDa classic myelin basic protein (MBP) is an intrinsically disordered protein arising from the Golli (Genes of Oligodendrocyte Lineage) gene complex and is responsible for compaction of the myelin sheath in the central nervous system. This MBP splice isoform also has a plethora of post-translational modifications including phosphorylation, deimination, methylation, and deamidation, that reduce its overall net charge and alter its protein and lipid associations within oligodendrocytes (OLGs). It was originally thought that MBP was simply a structural component of myelin; however, additional investigations have demonstrated that MBP is multi-functional, having numerous protein-protein interactions with Ca2+-calmodulin, actin, tubulin, and proteins with SH3-domains, and it can tether these proteins to a lipid membrane in vitro. Here, we have examined cytoskeletal interactions of classic 18.5-kDa MBP, in vivo, using early developmental N19-OLGs transfected with fluorescently-tagged MBP, actin, tubulin, and zonula occludens 1 (ZO-1). We show that MBP redistributes to distinct ‘membrane-ruffled’ regions of the plasma membrane where it co-localizes with actin and tubulin, and with the SH3-domain-containing proteins cortactin and ZO-1, when stimulated with PMA, a potent activator of the protein kinase C pathway. Moreover, using phospho-specific antibody staining, we show an increase in phosphorylated Thr98 MBP (human sequence numbering) in membrane-ruffled OLGs. Previously, Thr98 phosphorylation of MBP has been shown to affect its conformation, interactions with other proteins, and tethering of other proteins to the membrane in vitro. Here, MBP and actin were also co-localized in new focal adhesion contacts induced by IGF-1 stimulation in cells grown on laminin-2. This study supports a role for classic MBP isoforms in cytoskeletal and other protein-protein interactions during membrane and cytoskeletal remodeling in OLGs.









Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DMEM:
-
Dulbecco’s Modified Eagle Medium
- GFP:
-
Green fluorescent protein
- Golli:
-
Gene of oligodendrocyte lineage
- IGF-1:
-
Insulin-like growth factor-1
- MBP:
-
Myelin basic protein
- MAGUK:
-
Membrane-associated guanylate kinase
- NGS:
-
Normal goat serum
- OLG:
-
Oligodendrocyte
- OPC:
-
Oligodendroglial progenitor cell
- PBS:
-
Phosphate-buffered saline
- PKC:
-
Protein kinase C
- PMA:
-
Phorbol-12-myristate-13-acetate
- PNS:
-
Peripheral nervous system
- PSD-95:
-
Post-synaptic density protein of 95 kDa
- RFP:
-
Red fluorescent protein
- TIRF:
-
Total internal reflection fluorescence
- UTR:
-
Untranslated region
- ZO-1:
-
Zona occludens 1
References
Baumann N, Pham-Dinh D (2001) Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81:871–927
Sherman DL, Brophy PJ (2005) Mechanisms of axon ensheathment and myelin growth. Nat Rev Neurosci 6:683–690
Bradl M, Lassmann H (2010) Oligodendrocytes: biology and pathology. Acta Neuropathol 119:37–53
Miron VE, Kuhlmann T, Antel JP (2011) Cells of the oligodendroglial lineage, myelination, and remyelination. Biochim Biophys Acta 1812:184–193
Sobottka B, Ziegler U, Kaech A, Becher B, Goebels N (2011) CNS live imaging reveals a new mechanism of myelination: the liquid croissant model. Glia 59:1841–1849. doi:10.1002/glia.21228
Aggarwal S, Yurlova L, Simons M (2011) Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol 21:585–593
Fulton D, Paez PM, Campagnoni AT (2010) The multiple roles of myelin protein genes during the development of the oligodendrocyte. ASN Neuro 2:e00027
Boggs JM (2006) Myelin basic protein: a multifunctional protein. Cell Mol Life Sci 63:1945–1961
Fitzner D, Schneider A, Kippert A, Mobius W, Willig KI, Hell SW, Bunt G, Gaus K, Simons M (2006) Myelin basic protein-dependent plasma membrane reorganization in the formation of myelin. EMBO J 25:5037–5048
Boggs JM (2008) Myelin basic protein. Nova Science Publishers, Hauppauge, NY
Harauz G, Libich DS (2009) The classic basic protein of myelin—conserved structural motifs and the dynamic molecular barcode involved in membrane adhesion and protein-protein interactions. Current Protein and Peptide Science 10:196–215
Harauz G, Ladizhansky V, Boggs JM (2009) Structural polymorphism and multifunctionality of myelin basic protein. Biochemistry 48:8094–8104
Aggarwal S, Yurlova L, Snaidero N, Reetz C, Frey S, Zimmermann J, Pahler G, Janshoff A, Friedrichs J, Muller DJ, Goebel C, Simons M (2011) A size barrier limits protein diffusion at the cell surface to generate lipid-rich myelin-membrane sheets. Dev Cell 21:445–456
Givogri MI, Bongarzone ER, Schonmann V, Campagnoni AT (2001) Expression and regulation of golli products of myelin basic protein gene during in vitro development of oligodendrocytes. J Neurosci Res 66:679–690
Harauz G, Ishiyama N, Hill CMD, Bates IR, Libich DS, Farès C (2004) Myelin basic protein—diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis. Micron 35:503–542
Polverini E, Rangaraj G, Libich DS, Boggs JM, Harauz G (2008) Binding of the proline-rich segment of myelin basic protein to SH3-domains—spectroscopic, microarray, and modelling studies of ligand conformation and effects of post-translational modifications. Biochemistry 47:267–282
Bamm VV, Ahmed MA, Harauz G (2010) Interaction of myelin basic protein with actin in the presence of dodecylphosphocholine micelles. Biochemistry 49:6903–6915
Bamm VV, De Avila M, Smith GST, Ahmed MA, Harauz G (2011) Structured functional domains of myelin basic protein: cross talk between actin polymerization and Ca(2+)-dependent calmodulin interaction. Biophys J 101:1248–1256
Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM (1993) The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA 90:7834–7838
Li X, Ionescu AV, Lynn BD, Lu S, Kamasawa N, Morita M, Davidson KG, Yasumura T, Rash JE, Nagy JI (2004) Connexin47, connexin29 and connexin32 co-expression in oligodendrocytes and Cx47 association with zonula occludens-1 (ZO-1) in mouse brain. Neuroscience 126:611–630
Penes MC, Li X, Nagy JI (2005) Expression of zonula occludens-1 (ZO-1) and the transcription factor ZO-1-associated nucleic acid-binding protein (ZONAB)-MsY3 in glial cells and colocalization at oligodendrocyte and astrocyte gap junctions in mouse brain. Eur J Neurosci 22:404–418
Alanne MH, Pummi K, Heape AM, Grenman R, Peltonen J, Peltonen S (2009) Tight junction proteins in human Schwann cell autotypic junctions. J Histochem Cytochem 57:523–529
Boggs JM, Rangaraj G, Hill CMD, Bates IR, Heng YM, Harauz G (2005) Effect of arginine loss in myelin basic protein, as occurs in its deiminated charge isoform, on mediation of actin polymerization and actin binding to a lipid membrane in vitro. Biochemistry 44:3524–3534
Hill CMD, Harauz G (2005) Charge effects modulate actin assembly by classic myelin basic protein isoforms. Biochem Biophys Res Commun 329:362–369
Boggs JM, Rangaraj G (2000) Interaction of lipid-bound myelin basic protein with actin filaments and calmodulin. Biochemistry 39:7799–7806
Boggs JM, Rangaraj G, Heng YM, Liu Y, Harauz G (2011) Myelin basic protein binds microtubules to a membrane surface and to actin filaments in vitro: effect of phosphorylation and deimination. Biochim Biophys Acta 1808:761–773
Hill CMD, Libich DS, Harauz G (2005) Assembly of tubulin by classic myelin basic protein isoforms and regulation by post-translational modification. Biochemistry 44:16672–16683
Pirollet F, Derancourt J, Haiech J, Job D, Margolis RL (1992) Ca(2+)-calmodulin regulated effectors of microtubule stability in bovine brain. Biochemistry 31:8849–8855
Galiano MR, Andrieux A, Deloulme JC, Bosc C, Schweitzer A, Job D, Hallak ME (2006) Myelin basic protein functions as a microtubule stabilizing protein in differentiated oligodendrocytes. J Neurosci Res 84:534–541
Dyer CA, Philibotte TM, Billings-Gagliardi S, Wolf MK (1995) Cytoskeleton in myelin-basic-protein-deficient shiverer oligodendrocytes. Dev Neurosci 17:53–62
Richter-Landsberg C (2001) Organization and functional roles of the cytoskeleton in oligodendrocytes. Microsc Res Tech 52:628–636
Richter-Landsberg C (2008) The cytoskeleton in oligodendrocytes: microtubule dynamics in health and disease. J Mol Neurosci 35:55–63
Bauer NG, Richter-Landsberg C, Ffrench-Constant C (2009) Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia 57:1691–1705
Homchaudhuri L, Polverini E, Gao W, Harauz G, Boggs JM (2009) Influence of membrane surface charge and post-translational modifications to myelin basic protein on its ability to tether the Fyn-SH3 domain to a membrane in vitro. Biochemistry 48:2385–2393
Smith GS, De AM, Paez PM, Spreuer V, Wills MK, Jones N, Boggs JM, Harauz G (2012) Proline substitutions and threonine pseudophosphorylation of the SH3 ligand of 18.5-kDa myelin basic protein decrease its affinity for the Fyn-SH3 domain and alter process development and protein localization in oligodendrocytes. J Neurosci Res 90:28–47
De Avila M, Ahmed MAM, Smith GST, Boggs JM, Harauz G (2011) Modes of SH3-domain interactions of 18.5 kDa myelin basic protein in vitro and in oligodendrocytes. Biophysical Journal, Abstract B164, 55th annual meeting of the Biophysical Society, Baltimore, MD, 5–9 March 2011
Bates IR, Libich DS, Wood DD, Moscarello MA, Harauz G (2002) An Arg/Lys → Gln mutant of recombinant murine myelin basic protein as a mimic of the deiminated form implicated in multiple sclerosis. Protein Expr Purif 25:330–341
Smith GST, Paez PM, Spreuer V, Campagnoni CW, Boggs JM, Campagnoni AT, Harauz G (2011) Classical 18.5- and 21.5-kDa isoforms of myelin basic protein inhibit calcium influx into oligodendroglial cells, in contrast to golli isoforms. J Neurosci Res 89:467–480
Riesen FK, Rothen-Rutishauser B, Wunderli-Allenspach H (2002) A ZO1-GFP fusion protein to study the dynamics of tight junctions in living cells. Histochem Cell Biol 117:307–315
Bates IR, Matharu P, Ishiyama N, Rochon D, Wood DD, Polverini E, Moscarello MA, Viner NJ, Harauz G (2000) Characterization of a recombinant murine 18.5-kDa myelin basic protein. Protein Expr Purif 20:285–299
Guilak F (1994) Volume and surface area measurement of viable chondrocytes in situ using geometric modelling of serial confocal sections. J Microsc 173:245–256
Zhu Q, Tekola P, Baak JP, Belien JA (1994) Measurement by confocal laser scanning microscopy of the volume of epidermal nuclei in thick skin sections. Anal Quant Cytol Histol 16:145–152
Galvin J, Eyermann C, Colognato H (2010) Dystroglycan modulates the ability of insulin-like growth factor-1 to promote oligodendrocyte differentiation. J Neurosci Res 88:3295–3307
Verity AN, Bredesen D, Vonderscher C, Handley VW, Campagnoni AT (1993) Expression of myelin protein genes and other myelin components in an oligodendrocytic cell line conditionally immortalized with a temperature-sensitive retrovirus. J Neurochem 60:577–587
Foster LM, Phan T, Verity AN, Bredesen D, Campagnoni AT (1993) Generation and analysis of normal and shiverer temperature-sensitive immortalized cell lines exhibiting phenotypic characteristics of oligodendrocytes at several stages of differentiation. Dev Neurosci 15:100–109
Paez PM, Spreuer V, Handley V, Feng JM, Campagnoni C, Campagnoni AT (2007) Increased expression of golli myelin basic proteins enhances calcium influx into oligodendroglial cells. J Neurosci 27:12690–12699
Jacobs EC, Reyes SD, Campagnoni CW, Givogri I, Kampf K, Handley V, Spreuer V, Fisher R, Macklin W, Campagnoni AT (2009) Targeted overexpression of a golli-myelin basic protein isoform to oligodendrocytes results in aberrant oligodendrocyte maturation and myelination. ASN Neuro 1:e00017
Perez MJ, Ortiz EH, Roffe M, Soto EF, Pasquini JM (2009) Fyn kinase is involved in oligodendroglial cell differentiation induced by apotransferrin. J Neurosci Res 87:3378–3389
Ainger K, Avossa D, Diana AS, Barry C, Barbarese E, Carson JH (1997) Transport and localization elements in myelin basic protein mRNA. J Cell Biol 138:1077–1087
Carson JH, Gao Y, Tatavarty V, Levin MK, Korza G, Francone VP, Kosturko LD, Maggipinto MJ, Barbarese E (2008) Multiplexed RNA trafficking in oligodendrocytes and neurons. Biochim Biophys Acta 1779:453–458
Pedraza L, Fidler L, Staugaitis SM, Colman DR (1997) The active transport of myelin basic protein into the nucleus suggests a regulatory role in myelination. Neuron 18:579–589
Pedraza L (1997) Nuclear transport of myelin basic protein. J Neurosci Res 50:258–264
Liu DY, Martic M, Grkovic I, Garrett C, Dunlop ME, Baker HW (2002) Phorbol myristate acetate induces ruffling of the acrosome of human sperm. Fertil Steril 78:128–136
di Campli A, Valderrama F, Babia T, De Matteis MA, Luini A, Egea G (1999) Morphological changes in the Golgi complex correlate with actin cytoskeleton rearrangements. Cell Motil Cytoskelet 43:334–348
Stariha RL, Kikuchi S, Siow YL, Pelech SL, Kim M, Kim SU (1997) Role of extracellular signal-regulated protein kinases 1 and 2 in oligodendroglial process extension. J Neurochem 68:945–953
Erickson AK, Payne DM, Martino PA, Rossomando AJ, Shabanowitz J, Weber MJ, Hunt DF, Sturgill TW (1990) Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J Biol Chem 265:19728–19735
Yu JS, Yang SD (1994) Protein kinase FA/glycogen synthase kinase-3 predominantly phosphorylates the in vivo site Thr97-Pro in brain myelin basic protein: evidence for Thr-Pro and Ser-Arg-X-X-Ser as consensus sequence motifs. J Neurochem 62:1596–1603
Feldman EL, Sullivan KA, Kim B, Russell JW (1997) Insulin-like growth factors regulate neuronal differentiation and survival. Neurobiol Dis 4:201–214
Cheng HL, Steinway ML, Russell JW, Feldman EL (2000) GTPases and phosphatidylinositol 3-kinase are critical for insulin-like growth factor-I-mediated Schwann cell motility. J Biol Chem 275:27197–27204
Manneville JB (2006) Use of TIRF microscopy to visualize actin and microtubules in migrating cells. Methods Enzymol 406:520–532
Grigoriev I, Akhmanova A (2010) Microtubule dynamics at the cell cortex probed by TIRF microscopy. Methods Cell Biol 97:91–109
Webb RL, Rozov O, Watkins SC, McCartney BM (2009) Using total internal reflection fluorescence (TIRF) microscopy to visualize cortical actin and microtubules in the drosophila syncytial embryo. Dev Dyn 238:2622–2632
Axelrod D (2008) Chapter 7: total internal reflection fluorescence microscopy. Methods Cell Biol 89:169–221
Ammer AG, Weed SA (2008) Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton 65:687–707
Song J, Goetz BD, Baas PW, Duncan ID (2001) Cytoskeletal reorganization during the formation of oligodendrocyte processes and branches. Mol Cell Neurosci 17:624–636
Tsukada M, Prokscha A, Ungewickell E, Eichele G (2005) Doublecortin association with actin filaments is regulated by neurabin II. J Biol Chem 280:11361–11368
Klein C, Kramer EM, Cardine AM, Schraven B, Brandt R, Trotter J (2002) Process outgrowth of oligodendrocytes is promoted by interaction of Fyn kinase with the cytoskeletal protein tau. J Neurosci 22:698–707
Dyer CA, Benjamins JA (1989) Organization of oligodendroglial membrane sheets. I: association of myelin basic protein and 2′,3′-cyclic nucleotide 3′-phosphohydrolase with cytoskeleton. J Neurosci Res 24:201–211
DeBruin LS, Haines JD, Wellhauser LA, Radeva G, Schonmann V, Bienzle D, Harauz G (2005) Developmental partitioning of myelin basic protein into membrane microdomains. J Neurosci Res 80:211–225
Arvanitis DN, Min W, Gong Y, Heng YM, Boggs JM (2005) Two types of detergent-insoluble, glycosphingolipid/cholesterol-rich membrane domains from isolated myelin. J Neurochem 94:1696–1710
Karthigasan J, Kosaras B, Nguyen J, Kirschner DA (1994) Protein and lipid composition of radial component-enriched CNS myelin. J Neurochem 62:1203–1213
Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S (1999) Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol 145:579–588
DeBruin LS, Harauz G (2007) White matter rafting—membrane microdomains in myelin. Neurochem Res 32:213–228
Polverini E, Coll EP, Tieleman DP, Harauz G (2011) Conformational choreography of a molecular switch region in myelin basic protein-molecular dynamics shows induced folding and secondary structure type conversion upon threonyl phosphorylation in both aqueous and membrane-associated environments. Biochim Biophys Acta 1808:674–683
Bessonov K, Bamm VV, Harauz G (2010) Misincorporation of the proline homologue Aze (azetidine-2-carboxylic acid) into recombinant myelin basic protein. Phytochemistry 71:502–507
Atkins CM, Yon M, Groome NP, Sweatt JD (1999) Regulation of myelin basic protein phosphorylation by mitogen-activated protein kinase during increased action potential firing in the hippocampus. J Neurochem 73:1090–1097
Kim JK, Mastronardi FG, Wood DD, Lubman DM, Zand R, Moscarello MA (2003) Multiple sclerosis: an important role for post-translational modifications of myelin basic protein in pathogenesis. Mol Cell Proteomics 2:453–462
Schulz P, Cruz TF, Moscarello MA (1988) Endogenous phosphorylation of basic protein in myelin of varying degrees of compaction. Biochemistry 27:7793–7799
DeBruin LS, Haines JD, Bienzle D, Harauz G (2006) Partitioning of myelin basic protein into membrane microdomains in a spontaneously demyelinating mouse model for multiple sclerosis. Biochem Cell Biol 84:993–1005
Medveczky P, Antal J, Patthy A, Kekesi K, Juhasz G, Szilagyi L, Graf L (2006) Myelin basic protein, an autoantigen in multiple sclerosis, is selectively processed by human trypsin 4. FEBS Lett 580:545–552
Deibler GE, Stone AL, Kies MW (1990) Role of phosphorylation in conformational adaptability of bovine myelin basic protein. Proteins 7:32–40
Ramwani JJ, Epand RM, Moscarello MA (1989) Secondary structure of charge isomers of myelin basic protein before and after phosphorylation. Biochemistry 28:6538–6543
Boggs JM, Rangaraj G, Gao W, Heng YM (2006) Effect of phosphorylation of myelin basic protein by MAPK on its interactions with actin and actin binding to a lipid membrane in vitro. Biochemistry 45:391–401
Wood DD, Moscarello MA (1989) The isolation, characterization, and lipid-aggregating properties of a citrulline containing myelin basic protein. J Biol Chem 264:5121–5127
Moscarello MA, Wood DD, Ackerley C, Boulias C (1994) Myelin in multiple sclerosis is developmentally immature. J Clin Invest 94:146–154
Homchaudhuri L, De AM, Nilsson SB, Bessonov K, Smith GS, Bamm VV, Musse AA, Harauz G, Boggs JM (2010) Secondary structure and solvent accessibility of a calmodulin-binding C-terminal segment of membrane-associated myelin basic protein. Biochemistry 49:8955–8966
Karthigasan J, Garvey JS, Ramamurthy GV, Kirschner DA (1996) Immunolocalization of 17 and 21.5 kDa MBP isoforms in compact myelin and radial component. J Neurocytol 25:1–7
Baron W, Hoekstra D (2010) On the biogenesis of myelin membranes: sorting, trafficking and cell polarity. FEBS Lett 584:1760–1770
Maier O, Hoekstra D, Baron W (2008) Polarity development in oligodendrocytes: sorting and trafficking of myelin components. J Mol Neurosci 35:35–53
Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE (2003) Tight junction proteins. Prog Biophys Mol Biol 81:1–44
Tsukita S, Furuse M (2002) Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol 14:531–536
Maglione M, Tress O, Haas B, Karram K, Trotter J, Willecke K, Kettenmann H (2010) Oligodendrocytes in mouse corpus callosum are coupled via gap junction channels formed by connexin47 and connexin32. Glia 58:1104–1117
Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273:29745–29753
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MOP #86483, J.M.B. and G.H.), and the Natural Sciences and Engineering Research Council of Canada (RG121541 to G.H.). G.H. is a Tier I Canada Research Chair. G.S.T.S. is a recipient of a Doctoral Studentship from the Multiple Sclerosis Society of Canada. The pGEX2T-ZO-1-SH3 plasmid was a kind gift from Dr. Maria Balda (University College London, UK). The pGFP-ZO-1 plasmid was a kind gift from Dr. Heidi Wunderli-Allenspach (Institute of Pharmaceutical Sciences, ETH-Zürich). The authors are grateful to Dr. Vladimir Bamm for many helpful discussions, Ms. Sara Gagnon and Ms. Lindsay Petley-Ragan for their technical assistance in the early stages of this project, and to Dr. Reihua (Ray) Lu for generous use of his epifluorescence microscope.
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue: In honor of Dr. Bob Ledeen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11064_2011_700_MOESM1_ESM.tif
Supplementary Figure 1. Fluorescent intensity profile analyses of RFP-MBP-C1-UTR (red) within membrane-ruffled regions of N19-OLGs when compared to β-actin, γ-actin, α-tubulin, and the SH3-domain-containing proteins cortactin and ZO-1 following 5 min stimulation with PMA. Areas in dashed squares are shown magnified on the right. The yellow lines in each image indicate the area within the ruffled regions that was examined for intensity. Increases in co-localization of MBP with cytoskeletal proteins can be observed for each treatment as indicated by the intensity profiles. Identical observations were observed with RFP-MBP-C8-UTR and RFP-MBP-21.5-UTR (not shown). Scale bar = 20 μm (TIFF 18829 kb)
11064_2011_700_MOESM2_ESM.tif
Supplementary Figure 2. Three-dimensional reconstructions of 0.3 μm serial images of N19-OLGs treated with PMA, demonstrating an enrichment of RFP-MBP-C1-UTR (red) in membrane-ruffled regions when compared to β-actin, γ-actin, α-tubulin, and the SH3-domain-containing proteins cortactin and ZO-1, following a 5 min stimulation using PMA. Increases in co-localization of MBP and these cytoskeletal proteins can be observed along ruffled membrane edges (yellow, white arrowheads) of OLGs compared to GFP alone. Images for each PMA-stimulation of MBP are provided from both a vertical and angular perspective to help distinguish structural changes of the ruffled plasma membrane. Scale bar = 20 μm (TIFF 18403 kb)
Video 1. Transfected N19-OLGs responding to PMA treatment showing dynamics of RFP-MBP-C1-UTR (red) and GFP-β-actin (green) over a 10 min time course. MBP and actin show co-localization within membrane-ruffled regions at the periphery of the cell and throughout the cell body. Images for each channel were acquired every 10 s, and the frame rate of the video was set at 10 frames/s. Scale bar = 10 μm (Video 1) or 5 μm (Video 2) (MP4 238 kb)
Video 2. Transfected N19-OLGs responding to PMA treatment showing dynamics of RFP-MBP-C1-UTR (red) and GFP-β-actin (green) over a 10 min time course. MBP and actin show co-localization within membrane-ruffled regions at the periphery of the cell and throughout the cell body. Images for each channel were acquired every 10 s, and the frame rate of the video was set at 10 frames/s. Scale bar = 10 μm (Video 1) or 5 μm (Video 2) (MP4 283 kb)
Video 3. Transfected N19-OLGs responding to PMA treatment showing dynamics of RFP-MBP-C1-UTR (red) and GFP-α-tubulin (green) over a 6.5 min time course. The MBP and α-tubulin show co-localization within membrane-ruffled regions at the periphery of the cell and throughout the cell body. Images for each channel were acquired every 10 s, and the frame rate of the video was set at 10 frames/s. Scale bar = 10 μm (Video 3) or 5 μm (Video 4) (MP4 81 kb)
Video 4. Transfected N19-OLGs responding to PMA treatment showing dynamics of RFP-MBP-C1-UTR (red) and GFP-α-tubulin (green) over a 6.5 min time course. The MBP and α-tubulin show co-localization within membrane-ruffled regions at the periphery of the cell and throughout the cell body. Images for each channel were acquired every 10 s, and the frame rate of the video was set at 10 frames/s. Scale bar = 10 μm (Video 3) or 5 μm (Video 4) (MP4 82 kb)
Video 5. Transfected N19-OLGs responding to PMA treatment showing dynamics of RFP-MBP-21.5-UTR (red) and GFP-β-actin (green) over a 10 min time course. The MBP and actin show co-localization within membrane-ruffled regions at the periphery of the cell and throughout the cell body. The white arrows show membrane-ruffled regions, whereas the blue arrows show active areas of actin polymerization and co-localization with MBP. Images for each channel were acquired every 10 s, and the frame rate of the video was set at 10 frames/s. Scale bar = 20 μm. (MP4 741 kb)
Video 6. Transfected N19-OLGs responding to PMA treatment showing dynamics of RFP-MBP-C1-UTR (red) and GFP-ZO-1 (green) over a 8 min time course. The MBP and ZO-1 show co-localization within membrane-ruffled regions throughout the plasma membrane and cell body. Images for each channel were acquired every 10 s, and the frame rate of the video was set at 10 frames/s; highlighted pixels (white) show areas of co-localization of MBP and ZO-1. Scale bar = 20 μm (MP4 131 kb)
Rights and permissions
About this article
Cite this article
Smith, G.S.T., Homchaudhuri, L., Boggs, J.M. et al. Classic 18.5- and 21.5-kDa Myelin Basic Protein Isoforms Associate with Cytoskeletal and SH3-Domain Proteins in the Immortalized N19-Oligodendroglial Cell Line Stimulated by Phorbol Ester and IGF-1. Neurochem Res 37, 1277–1295 (2012). https://doi.org/10.1007/s11064-011-0700-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0700-2