Abstract
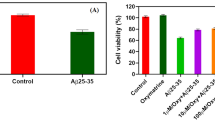
The deposition of amyloid β-protein (Aβ) fibrils into plaques within the brain parenchyma and along cerebral blood vessels is a hallmark of Alzheimer’s disease (AD). Aβ42 oligomers and fibrils cause the breakdown of neural circuits, neuronal death and eventually dementia. Drugs that inhibit Aβ42 aggregation may be a novel direction in AD drug discovery. Cryptotanshinone (CTS), an active component of the medicinal herb Salvia miltiorrhiza, has been shown to improve learning and memory in several pharmacological models of AD. However, the effects of CTS on the Aβ aggregation and toxicity are unclear. The current work shows the effectiveness of CTS on the inhibition of Aβ42 aggregation and toxicity to human neuroblastoma cells. In this study, we demonstrated that CTS can inhibit Aβ42 spontaneous aggregation using thioflavin T fluorescence assay and transmission electron microscopy. Furthermore, we investigated the effects of CTS on Aβ-induced oxidative cell death in cultured SH-SY5Y cells. MTT and lactate dehydrogenase assays showed that CTS reduced the cytotoxicity induced by Aβ42. CTS also dramatically reduced Aβ42-induced cellular apoptosis and increased level of reactive oxygen species in these cells. Our study suggests that CTS may be useful in the inhibition or prevention of AD development and progression.






Similar content being viewed by others
References
Hu X, Crick SL, Bu G, Frieden C, Pappu RV, Lee JM (2009) Amyloid seeds formed by cellular uptake, concentration, and aggregation of the amyloid-beta peptide. Proc Natl Acad Sci USA 106:20324–20329
Aderinwale OG, Ernst HW, Mousa SA (2010) Current therapies and new strategies for the management of Alzheimer’s disease. Am J Alzheimers Dis Other Demen 25(5):414–424
Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB (2003) Amyloid beta -protein (Abeta) assembly: Abeta 40 and Abeta 42 oligomerize through distinct pathways. Proc Natl Acad Sci USA 100:330–335
Allaman I, Gavillet M, Bélanger M, Laroche T, Viertl D, Lashuel HA, Magistretti PJ (2010) Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J Neurosci 30(9):3326–3338
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74
Yamin G, Ruchala P, Teplow DB (2009) A peptide hairpin inhibitor of amyloid beta-protein oligomerization and fibrillogenesis. Biochemistry 48:11329–11331
Riviere C, Delaunay JC, Immel F, Cullin C, Monti JP (2009) The polyphenol piceid destabilizes preformed amyloid fibrils and oligomers in vitro: hypothesis on possible molecular mechanisms. Neurochem Res 34:1120–1128
McLaurin J, Cecal R, Kierstead ME, Tian X, Phinney AL, Manea M, French JE, Lambermon MH, Darabie AA, Brown ME, Janus C, Chishti MA, Horne P, Westaway D, Fraser PE, Mount HT, Przybylski M, St George-Hyslop P (2002) Therapeutically effective antibodies against amyloid-beta peptide target amyloid-beta residues 4–10 and inhibit cytotoxicity and fibrillogenesis. Nat Med 8:1263–1269
Nishida Y, Ito S, Ohtsuki S, Yamamoto N, Takahashi T, Iwata N, Jishage K, Yamada H, Sasaguri H, Yokota S, Piao W, Tomimitsu H, Saido TC, Yanagisawa K, Terasaki T, Mizusawa H, Yokota T (2009) Depletion of vitamin E increases amyloid beta accumulation by decreasing its clearances from brain and blood in a mouse model of Alzheimer disease. J Biol Chem 284:33400–33408
Thomas T, Nadackal TG, Thomas K (2001) Aspirin and non-steroidal anti-inflammatory drugs inhibit amyloid-beta aggregation. Neuroreport 12:3263–3267
Jin YC, Kim CW, Kim YM, Nizamutdinova IT, Ha YM, Kim HJ, Seo HG, Son KH, Jeon SJ, Kang SS, Kim YS, Kam SC, Lee JH, Chang KC (2009) Cryptotanshinone, a lipophilic compound of Salvia miltiorrriza root, inhibits TNF-alpha-induced expression of adhesion molecules in HUVEC and attenuates rat myocardial ischemia/reperfusion injury in vivo. Eur J Pharmacol 614:91–97
Yin HQ, Choi YJ, Kim YC, Sohn DH, Ryu SY, Lee BH (2009) Salvia miltiorrhiza Bunge and its active component cryptotanshinone protects primary cultured rat hepatocytes from acute ethanol-induced cytotoxicity and fatty infiltration. Food Chem Toxicol 47:98–103
Wong KK, Ho MT, Lin HQ, Lau KF, Rudd JA, Chung RC, Fung KP, Shaw PC, Wan DC (2010) Cryptotanshinone, an acetylcholinesterase inhibitor from Salvia miltiorrhiza, ameliorates scopolamine-induced amnesia in Morris water maze task. Planta Med 76:228–234
Adams JD, Wang R, Yang J, Lien EJ (2006) Preclinical and clinical examinations of Salvia miltiorrhiza and its tanshinones in ischemic conditions. Chin Med 1:3
Yu XY, Lin SG, Chen X, Zhou ZW, Liang J, Duan W, Chowbay B, Wen JY, Chan E, Cao J, Li CG, Zhou SF (2007) Transport of cryptotanshinone, a major active triterpenoid in Salvia miltiorrhiza Bunge widely used in the treatment of stroke and Alzheimer’s disease, across the blood-brain barrier. Curr Drug Metab 8:365–378
Isaacs AM, Senn DB, Yuan M, Shine JP, Yankner BA (2006) Acceleration of amyloid beta-peptide aggregation by physiological concentrations of calcium. J Biol Chem 281:27916–27923
Polimeno L, Pesetti B, Lisowsky T, Iannone F, Resta L, Giorgio F, Mallamaci R, Buttiglione M, Santovito D, Vitiello F, Mancini ME, Francavilla A (2009) Protective effect of augmenter of liver regeneration on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Free Radic Res 43:865–875
Spallarossa P, Garibaldi S, Altieri P, Fabbi P, Manca V, Nasti S, Rossettin P, Ghigliotti G, Ballestrero A, Patrone F, Barsotti A, Brunelli C (2004) Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol 37:837–846
Wolfe MS (2002) Therapeutic strategies for Alzheimer’s disease. Nat Rev Drug Discov 1:859–866
Jana M, Palencia CA, Pahan K (2008) Fibrillar amyloid-beta peptides activate microglia via TLR2: implications for Alzheimer’s disease. J Immunol 181:7254–7262
Faller P (2009) Copper and zinc binding to amyloid-beta: coordination, dynamics, aggregation, reactivity and metal-ion transfer. Chembiochem 10:2837–2845
Grossi C, Francese S, Casini A, Rosi MC, Luccarini I, Fiorentini A, Gabbiani C, Messori L, Moneti G, Casamenti F (2009) Clioquinol decreases amyloid-beta burden and reduces working memory impairment in a transgenic mouse model of Alzheimer’s disease. J Alzheimers Dis 17:423–440
Choo-Smith LP, Garzon-Rodriguez W, Glabe CG, Surewicz WK (1997) Acceleration of amyloid fibril formation by specific binding of Abeta-(1–40) peptide to ganglioside-containing membrane vesicles. J Biol Chem 272:22987–22990
Iurescia S, Fioretti D, Mangialasche F, Rinaldi M (2010) The pathological cross talk between apolipoprotein E and amyloid-beta peptide in Alzheimer’s disease: emerging gene-based therapeutic approaches. J Alzheimers Dis 21(1):35–48
McLaurin J, Franklin T, Zhang X, Deng J, Fraser PE (1999) Interactions of Alzheimer amyloid-beta peptides with glycosaminoglycans effects on fibril nucleation and growth. Eur J Biochem 266:1101–1110
Mei Z, Zhang F, Tao L, Zheng W, Cao Y, Wang Z, Tang S, Le K, Chen S, Pi R, Liu P (2009) Cryptotanshinone, a compound from Salvia miltiorrhiza modulates amyloid precursor protein metabolism and attenuates beta-amyloid deposition through upregulating alpha-secretase in vivo and in vitro. Neurosci Lett 452(2):90–95
Skovronsky DM, Doms RW, Lee VM (1998) Detection of a novel intraneuronal pool of insoluble amyloid beta protein that accumulates with time in culture. J Cell Biol 141:1031–1039
Vaisid T, Kosower NS, Elkind E, Barnoy S (2008) Amyloid beta peptide toxicity in differentiated PC12 cells: calpain-calpastatin, caspase, and membrane damage. J Neurosci Res 86:2314–2325
Lloret A, Badia MC, Mora NJ, Ortega A, Pallardo FV, Alonso MD, Atamna H, Vina J (2008) Gender and age-dependent differences in the mitochondrial apoptogenic pathway in Alzheimer’s disease. Free Radic Biol Med 44:2019–2025
Miranda S, Opazo C, Larrondo LF, Munoz FJ, Ruiz F, Leighton F, Inestrosa NC (2000) The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog Neurobiol 62:633–648
Boldogh I, Kruzel ML (2008) Colostrinin: an oxidative stress modulator for prevention and treatment of age-related disorders. J Alzheimers Dis 13:303–321
Moon JH, Kim SY, Lee HG, Kim SU, Lee YB (2008) Activation of nicotinic acetylcholine receptor prevents the production of reactive oxygen species in fibrillar beta amyloid peptide (1–42)-stimulated microglia. Exp Mol Med 40:11–18
Bras M, Queenan B, Susin SA (2005) Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 70:231–239
Pelicano H, Feng L, Zhou Y, Carew JS, Hileman EO, Plunkett W, Keating MJ, Huang P (2003) Inhibition of mitochondrial respiration: a novel strategy to enhance drug-induced apoptosis in human leukemia cells by a reactive oxygen species-mediated mechanism. J Biol Chem 278:37832–37839
Acknowledgments
This work was supported in part by Major programin key field of the Government of Guangdong Province, China (No. 2003A30904), NSFC-CIHR (No.30811120434) and The Ministry of Science and Technology of China,major special project—”significant Creation of new drugs”,No 2009ZX09102-152 and No.2009ZX09303-007 the National Natural Science Foundation (grant No.30500574).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mei, Z., Yan, P., Situ, B. et al. Cryptotanshinione Inhibits β-Amyloid Aggregation and Protects Damage from β-Amyloid in SH-SY5Y Cells. Neurochem Res 37, 622–628 (2012). https://doi.org/10.1007/s11064-011-0652-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-011-0652-6