Abstract
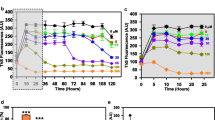
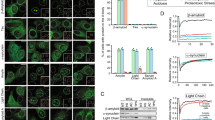
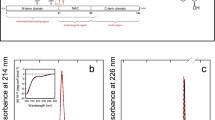
Alzheimer’s disease (AD) is characterized by deposits of amyloid in various tissues. The neuronal cytotoxicity of Aβ peptides is attributed not only to various mechanisms but also to amyloid fibrils and soluble oligomeric intermediates. Consequently, finding molecules to prevent or reverse the oligomerization and fibrillization of Aβ could be of therapeutic value in the treatment of AD. We show that piceid, a polyphenol of the stilbene family, destabilized fibrils and oligomers to give back monomers that are not neurotoxic molecules. The mechanism of this destabilization could be a dynamic interaction between the polyphenol and the Aβ that could open the hydrophobic zipper and shift the reversible equilibrium “random coil⇔β-sheet” to the disordered structure.







Similar content being viewed by others
References
Selkoe DJ (1997) Alzheimer’s disease: genotypes, phenotypes, and treatments. Science 275:630–631. doi:10.1126/science.275.5300.630
Murphy RM (2007) Kinetics of amyloid formation and membrane interaction with amyloidogenic proteins. Biochim Biophys Acta 1768:1923–1934. doi:10.1016/j.bbamem.2006.12.014 and references therein
Golde TE (2006) Disease modifying therapy for AD? J Neurochem 99:689–707. doi:10.1111/j.1471-4159.2006.04211.x
Rochet JC, Lansbury PT Jr (2000) Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol 10:60–68. doi:10.1016/S0959-440X(99)00049-4 and references therein
Monji A, Utsumi H, Ueda T et al (2001) The relationship between the aggregational state of the amyloid-beta peptides and free radical generation by the peptides. J Neurochem 77:1425–1432. doi:10.1046/j.1471-4159.2001.00392.x
Sultana R, Newman S, Mohmmad-Abdul H et al (2004) Protective effect of the xanthate, D609, on Alzheimer’s amyloid beta-peptide (1–42)-induced oxidative stress in primary neuronal cells. Free Radic Res 38:449–458. doi:10.1080/1071576042000206478
Tabner BJ, El-Agnaf OM, Turnbull S et al (2005) Hydrogen peroxide is generated during the very early stages of aggregation of the amyloid peptides implicated in Alzheimer disease and familial British dementia. J Biol Chem 280:35789–35792. doi:10.1074/jbc.C500238200
Kagan BL, Hirakura Y, Azimov R et al (2002) The channel hypothesis of Alzheimer’s disease: current status. Peptides 23:1311–1315. doi:10.1016/S0196-9781(02)00067-0
Lauderback CM, Kanski J, Hackett JM et al (2002) Apolipoprotein E modulates Alzheimer’s Abeta(1–42)-induced oxidative damage to synaptosomes in an allele-specific manner. Brain Res 924:90–97. doi:10.1016/S0006-8993(01)03228-0
Oppermann UC, Salim S, Tjernberg LO et al (1999) Binding of amyloid beta-peptide to mitochondrial hydroxyacyl-CoA dehydrogenase (ERAB): regulation of an SDR enzyme activity with implications for apoptosis in Alzheimer’s disease. FEBS Lett 451:238–242. doi:10.1016/S0014-5793(99)00586-4
Holtzman DM (2004) In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J Mol Neurosci 23:247–254. doi:10.1385/JMN:23:3:247
Sadowski M, Pankiewicz J, Scholtzova H et al (2004) A synthetic peptide blocking the apolipoprotein E/beta-amyloid binding mitigates beta-amyloid toxicity and fibril formation in vitro and reduces beta-amyloid plaques in transgenic mice. Am J Pathol 165:937–948
Lustbader JW, Cirilli M, Lin C et al (2004) ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 304:448–452. doi:10.1126/science.1091230
Selkoe DJ (1999) Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature 399:A23–A31. doi:10.1038/19866
Yankner BA (1996) Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron 16:921–932. doi:10.1016/S0896-6273(00)80115-4
Pike CJ, Walencewicz AJ, Glabe CG et al (1991) In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res 563:311–314. doi:10.1016/0006-8993(91)91553-D
Pike CJ, Burdick D, Walencewicz AJ et al (1993) Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci 13:1676–1687
Lorenzo A, Yankner BA (1994) Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci USA 91:12243–12247. doi:10.1073/pnas.91.25.12243
Selkoe DJ (2004) Cell biology of protein misfolding: the examples of Alzheimer’s and Parkinson’s diseases. Nat Cell Biol 6:1054–1061. doi:10.1038/ncb1104-1054
Lambert MP, Barlow AK, Chromy BA et al (1998) Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95:6448–6453. doi:10.1073/pnas.95.11.6448
Walsh DM, Hartley DM, Kusumoto Y et al (1999) Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem 274:25945–25952. doi:10.1074/jbc.274.36.25945
Bhatia R, Lin H, Lal R (2000) Fresh and globular amyloid beta protein (1–42) induces rapid cellular degeneration: evidence for AbetaP channel-mediated cellular toxicity. FASEB J 14:1233–1243
Zhu YJ, Lin H, Lal R (2000) Fresh and nonfibrillar amyloid beta protein(1–40) induces rapid cellular degeneration in aged human fibroblasts: evidence for AbetaP-channel-mediated cellular toxicity. FASEB J 14:1244–1254
Klein WL, Krafft GA, Finch CE (2001) Targeting small Abeta oligomers: the solution to an Alzheimer’s disease conundrum? Trends Neurosci 24:219–224. doi:10.1016/S0166-2236(00)01749-5
Kim HJ, Chae SC, Lee DK et al (2003) Selective neuronal degeneration induced by soluble oligomeric amyloid beta protein. FASEB J 17:118–120
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297:353–356. doi:10.1126/science.1072994 Erratum in: Science. 2002. 297:2209 and comment in: Science. 2002 298:962–964
Kayed R, Head E, Thompson JL et al (2003) Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 300:486–489. doi:10.1126/science.1079469
Ono K, Yoshiike Y, Taskashima A et al (2003) Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem 87:172–181. doi:10.1046/j.1471-4159.2003.01976.x
Ono K, Hasegawa K, Naiki H et al (2004) Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J Neurosci Res 75:742–750. doi:10.1002/jnr.20025
Ono K, Hasegawa K, Naiki H et al (2004) Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Biochim Biophys Acta 1690:193–202
Rivière C, Richard T, Quentin L et al (2007) Inhibitory activity of stilbenes on Alzheimer’s beta-amyloid fibrils in vitro. Bioorg Med Chem 15:1160–1167. doi:10.1016/j.bmc.2006.09.069
Rivière C, Richard T, Vitrac X et al (2008) New polyphenols active on beta-amyloid aggregation. Bioorg Med Chem Lett 18:828–831. doi:10.1016/j.bmcl.2007.11.028
Yang F, Lim GP, Begum AN et al (2005) Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem 280:5892–5901. doi:10.1074/jbc.M404751200
Bastianetto S, Yao ZX, Papadopoulos V et al (2006) Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid-induced toxicity. Eur J NeuroSci 23:55–64. doi:10.1111/j.1460-9568.2005.04532.x
Hubbard GP, Wolffram S, Lovegrove JA et al (2003) The role of polyphenolic compounds in the diet as inhibitors of platelet function. Proc Nutr Soc 62:469–478. doi:10.1079/PNS2003253
Scalbert A, Manach C, Morand C et al (2005) Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr 45:287–306. doi:10.1080/1040869059096
Yang CS, Prabhu S, Landau J (2001) Prevention of carcinogenesis by tea polyphenols. Drug Metab Rev 33:237–253. doi:10.1081/DMR-120000651
Manach C, Scalbert A, Morand C et al (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Rossi L, Mazzitelli S, Arciello M et al. (2008) Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem Res (Published online)
Richard T, Verge S, Berke B et al (2001) NMR and simulated annealing investigations of bradykinin in presence of polyphenols. J Biomol Struct Dyn 18:627–637
Charlton AJ, Baxter NJ, Khan ML et al (2002) Polyphenol/peptide binding and precipitation. J Agric Food Chem 50:1593–1601. doi:10.1021/jf010897z
Charlton AJ, Haslam E, Williamson MP (2002) Multiple conformations of the proline-rich protein/epigallocatechin gallate complex determined by time-averaged nuclear Overhauser effects. J Am Chem Soc 124:9899–9905. doi:10.1021/ja0126374
Vergé S, Richard T, Moreau S et al (2002) First observation of solution structures of bradykinin-penta-O-galloyl-d-glucopyranose complexes as determined by NMR and simulated annealing. Biochim Biophys Acta 1571:89–101
Richard T, Vitrac X, Merillon JM et al (2005) Role of peptide primary sequence in polyphenol-protein recognition: an example with neurotensin. Biochim Biophys Acta 1726:238–243
Murray NJ, Williamson MP, Lilley TH et al (1994) Study of the interaction between salivary proline-rich proteins and a polyphenol by 1H-NMR spectroscopy. Eur J Biochem 219:923–935. doi:10.1111/j.1432-1033.1994.tb18574.x
Haslam E (1974) Polyphenol–protein interactions. Biochem J 139:285–288
Richard T, Delaunay JC, Merillon JM et al (2003) Is the C-terminal region of bradykinin the binding site of polyphenols? J Biomol Struct Dyn 21:379–385
Simon C, Barathieu K, Laguerre M et al (2003) Three-dimensional structure and dynamics of wine tannin-saliva protein complexes a multitechnique approach. Biochemistry 42:10385–10395. doi:10.1021/bi034354p
Klegeris A, Walker DG, McGeer PL (1994) Activation of macrophages by Alzheimer beta amyloid peptide. Biochem Biophys Res Commun 199:984–991. doi:10.1006/bbrc.1994.1326
Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J et al (1995) Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25–35 region to aggregation and neurotoxicity. J Neurochem 64:253–265
Hughes E, Burke RM, Doig AJ (2000) Inhibition of toxicity in the beta-amyloid peptide fragment beta-(25–35) using N-methylated derivatives: a general strategy to prevent amyloid formation. J Biol Chem 275:25109–25115. doi:10.1074/jbc.M003554200
Naiki H, Higuchi K, Hosokawa M et al (1989) Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye thioflavine T. Anal Biochem 177:244–249. doi:10.1016/0003-2697(89)90046-8
Chromy BA, Nowak RJ, Lambert MP et al (2003) Self-assembly of Abeta (1–42) into globular neurotoxins. Biochemistry 42:12749–12760. doi:10.1021/bi030029q
Atamna H, Boyle K (2006) Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc Natl Acad Sci USA 103:3381–3386. doi:10.1073/pnas.0600134103
Walsh DM, Lomakin A, Benedek GB et al (1997) Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem 272:22364–22372. doi:10.1074/jbc.272.35.22364
Chimon S, Shaibat MA, Jones CR et al (2007) Evidence of fibril-like beta-sheet structures in a neurotoxic amyloid intermediate of Alzheimer’s beta-amyloid. Nat Struct Mol Biol 14:1157–1164. doi:10.1038/nsmb1345
Pallitto MM, Murphy RM (2001) A mathematical model of the kinetics of beta-amyloid fibril growth from the denatured state. Biophys J 81:1805–1822
Hasegawa K, Ono K, Yamada M et al (2002) Kinetic modeling and determination of reaction constants of Alzheimer’s beta-amyloid fibril extension and dissociation using surface plasmon resonance. Biochemistry 41:13489–13498. doi:10.1021/bi020369w
Ono K, Hirohata M, Yamada M (2005) Ferulic acid destabilizes preformed beta-amyloid fibrils in vitro. Biochem Biophys Res Commun 336:444–449. doi:10.1016/j.bbrc.2005.08.148
Petkova AT, Ishii Y, Balbach JJ et al (2002) A structural model for Alzheimer’s beta-amyloid fibrils based on experimental constraints from solid state NMR. Proc Natl Acad Sci USA 99:16742–16747. doi:10.1073/pnas.262663499
Petkova AT, Yau WM, Tycko R (2006) Experimental constraints on quaternary structure in Alzheimer’s beta-amyloid fibrils. Biochemistry 45:498–512. doi:10.1021/bi051952q
Lührs T, Ritter C, Adrian M et al (2005) 3D structure of Alzheimer’s amyloid-beta(1–42) fibrils. Proc Natl Acad Sci USA 102:17342–17347. doi:10.1073/pnas.0506723102
Terzi E, Hölzemann G, Seelig J (1994) Alzheimer beta-amyloid peptide 25–35: electrostatic interactions with phospholipid membranes. Biochemistry 33:7434–7441. doi:10.1021/bi00189a051
Konno T (2001) Amyloid-induced aggregation and precipitation of soluble proteins: an electrostatic contribution of the Alzheimer’s beta (25–35) amyloid fibril. Biochemistry 40:2148–2154. doi:10.1021/bi002156h
Ippel JH, Olofsson A, Schleucher J et al (2002) Probing solvent accessibility of amyloid fibrils by solution NMR spectroscopy. Proc Natl Acad Sci USA 99:8648–8653. doi:10.1073/pnas.132098999
Kellermayer MS, Grama L, Karsai A et al (2005) Reversible mechanical unzipping of amyloid beta-fibrils. J Biol Chem 280:8464–8470. doi:10.1074/jbc.M411556200
Zeng H, Zhang Y, Peng L et al (2001) Nicotine and amyloid formation. Biol Psychiatry 49:248–257. doi:10.1016/S0006-3223(00)01111-2
Liu R, McAllister C, Lyubchenko Y et al (2004) Residues 17–20 and 30–35 of beta-amyloid play critical roles in aggregation. J Neurosci Res 75:162–171. doi:10.1002/jnr.10859
Pawar AP, Dubay KF, Zurdo J et al (2005) Prediction of “aggregation-prone” and “aggregation-susceptible” regions in proteins associated with neurodegenerative diseases. J Mol Biol 350:379–392. doi:10.1016/j.jmb.2005.04.016
Wei G, Shea JE (2006) Effects of solvent on the structure of the Alzheimer amyloid-beta(25–35) peptide. Biophys J 91:1638–1647. doi:10.1529/biophysj.105.079186 and references therein
Acknowledgments
We are grateful to SERCOMI for Electron Microscopy and to Dr. Ray Cooke for reading the manuscript. Financial support for this study came in part from the “Conseil Régional d’Aquitaine”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivière, C., Delaunay, JC., Immel, F. et al. The Polyphenol Piceid Destabilizes Preformed Amyloid Fibrils and Oligomers In Vitro: Hypothesis on Possible Molecular Mechanisms. Neurochem Res 34, 1120–1128 (2009). https://doi.org/10.1007/s11064-008-9883-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-008-9883-6