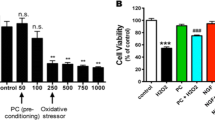
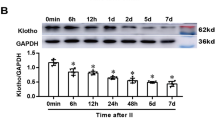
Abstract
Sublethal preconditioning ischemia protects neurons from lethal ischemia, and activation of ERK is associated with this protection. However, sublethal ischemia and reperfusion also results in rapid inactivation of ERK, which contributes to the dual-phase activation profile of ERK. In the present study, we observed sublethal ischemia-induced rapid inactivation of ERK was accompanied by phosphorylation of Raf-1 at Ser289/296/301 sites. Inhibition of calcium signaling by ketamine resulted in down-regulation of the Raf-1/ERK cascade and decreased phosphorylation of Raf-1 at Ser289/296/301. The MEK inhibitor U0126 suppressed ERK activity and phosphorylation of Raf-1 at Ser289/296/301 but not Raf-1 activation elicited by its dephosphorylation at S259 following ischemia. The PP2A inhibitor cantharidin but not Pin1 inhibitor juglone blocked Raf-1 dephosphorylation at Ser289/296/301 and ERK dephosphorylation and led to ERK sustained activation, which is associated with transcriptional up-regulation of genes related to differentiation. Furthermore, dual-phase activation of ERK did not alter the mRNA levels of genes related to proliferation or differentiation. These results indicate the initial robust activation of ERK phosphorylates Raf-1 at Ser289/296/301, resulting in Raf-1inhibition and then prompt inactivation of ERK following sublethal preconditioning ischemia. Dual-phase activation of ERK may exert its neuroprotection against lethal ischemia through blocking cell proliferation and differentiation.






Similar content being viewed by others
References
Pérez-Pinzón MA, Xu GP, Mumford PL et al (1997) Rapid ischemic preconditioning protects rats from cerebral anoxia/ischemia. Adv Exp Med Biol 428:155–161
Kitagawa K, Matsumoto M, Tagaya M et al (1990) ‘Ischemic tolerance’ phenomenon found in the brain. Brain Res 528(1):21–24
Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26(5):248–254
Gonzalez-Zulueta M, Feldman AB, Klesse LJ et al (1997) Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc Natl Acad Sci USA 97(1):436–441
Gu Z, Jiang Q, Zhang G (2001) Extracellular signal-regulated kinase and c-Jun N-terminal protein kinase in ischemic tolerance. Neuroreport 12(16):3487–3491
Lee S, Suk K, Kim IK, Jang IS, Park JW, Johnson VJ, Kwon TK, Choi BJ, Kim SH (2008) Signaling pathways of bisphenol A-induced apoptosis in hippocampal neuronal cells: role of calcium-induced reactive oxygen species, mitogen-activated protein kinases, and nuclear factor-kappaB. J Neurosci Res 86(13):2932–2942
Shamloo M, Rytter A, Wieloch T (1999) Activation of the extracellular signal-regulated protein kinase cascade in the hippocampal CA1 region in a rat model of global cerebral ischemic preconditioning. Neuroscience 93(1):81–88
Choi DW, Rothman SM (1990) The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci 13:171–182
Jantas D, Lason W (2009) Different mechanisms of NMDA-mediated protection against neuronal apoptosis: a stimuli-dependent effect. Neurochem Res 34:2040–2054
Mehta SL, Manhas N, Raghubir R (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 54(1):34–66
Moelling K, Schad K, Bosse M et al (2002) Regulation of Raf-Akt cross-talk. J Biol Chem 277(34):31099–31106
Sridhar SS, Hedley D, Siu LL (2005) Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther 4(4):677–685
Bełtowski J, Wójcicka G, Trzeciak J et al (2006) H2O2 and Src-dependent transactivation of the EGF receptor mediates the stimulatory effect of leptin on renal ERK and Na+, K+-ATPase. Peptides 27(12):3234–3244
Hekman M, Fischer A, Wennogle LP (2005) Novel C-Raf phosphorylation sites: serine 296 and 301 participate in Raf regulation. FEBS Lett 579(2):464–468
Dougherty MK, Müller J, Ritt DA et al (2005) Regulation of Raf-1 by direct feedback phosphorylation. Mol Cell 17(2):215–224
Ory S, Zhou M, Conrads TP et al (2003) Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14–3-3 binding sites. Curr Biol 13(16):1356–1364
Van Kanegan MJ, Adams DG, Wadzinski BE et al (2005) Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J Biol Chem 280(43):36029–36036
Miglietta A, Bozzo F, Gabriel L et al (2006) Extracellular signal-regulated kinase 1/2 and protein phosphatase 2A are involved in the antiproliferative activity of conjugated linoleic acid in MCF-7 cells. Br J Nutr 96(1):22–27
Zhao J, Wu HW, Chen YJ et al (2008) Protein phosphatase 2A-negative regulation of the protective signaling pathway of Ca2+/CaM-dependent ERK activation in cerebral ischemia. J Neurosci Res 86(12):2733–2745
Hu XH, Wu XY, Xu JL et al (2009) Src kinase up-regulates the ERK cascade through inactivation of protein phosphatase 2A following cerebral ischemia. BMC Neurosci 10(1):74
Sugino T, Nozaki K, Takagi Y et al (2000) Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus. J Neurosci 20(12):4506–4514
Sun X, Liu C, Qian M et al (2010) Ceramide from sphingomyelin hydrolysis differentially mediate mitogen-activated protein kinases (MAPKs) activation following cerebral ischemia in rat hippocampal CA1 subregion. J Biomed Res 24(2):46–51
Lee S, Yoon S, Kim DH (2007) A high nuclear basal level of ERK2 phosphorylation contributes to the resistance of cisplatin-resistant human ovarian cancer cells. Gynecol Oncol 104(2):338–344
Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80(2):179–185
Sasagawa S, Ozaki Y, Fujita K et al (2005) Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nat Cell Biol 7(4):365–373
Chalmers CJ, Gilley R, March HN et al (2007) The duration of ERK1/2 activity determines the activation of c-Fos and Fra-1 and the composition and quantitative transcriptional output of AP-1. Cell Signal 19(4):695–704
Yaka R, Gamliel A, Gurwitz D et al (1998) NGF induces transient but not sustained activation of ERK in PC12 mutant cells incapable of differentiating. J Cell Biochem 70(3):425–432
Sun P, Watanabe H, Takano K et al (2006) Sustained activation of M-Ras induced by nerve growth factor is essential for neuronal differentiation of PC12 cells. Genes Cells 11(9):1097–1113
Katz M, Amit I, Yarden Y (2007) Regulation of MAPKs by growth factors and receptor tyrosine kinases. Biochim Biophys Acta 1773(8):1161–1176
Pulsinelli WA, Brierley JB (1979) A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke 10(3):267–272
Chen XQ, Chen JG, Zhang Y et al (2003) 14–3-3gamma is upregulated by in vitro ischemia and binds to protein kinase Raf in primary cultures of astrocytes. Glia 42(4):315–324
Dumaz N, Marais R et al (2003) Protein kinase A blocks Raf-1 activity by stimulating 14–3-3 binding and blocking Raf-1 interaction with Ras. J Biol Chem 278(32):29819–29823
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (No. 30871200) and the Science and Technology Development Foundation of Nanjing Medical University (08NMUZ006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Q. Cao, M. Qian and X. F. Wang have contributed equally.
Rights and permissions
About this article
Cite this article
Cao, Q., Qian, M., Wang, X.F. et al. Negative Feedback Regulation of Raf/MEK/ERK Cascade After Sublethal Cerebral Ischemia in the Rat Hippocampus. Neurochem Res 36, 153–162 (2011). https://doi.org/10.1007/s11064-010-0285-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0285-1