Abstract
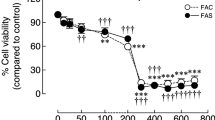
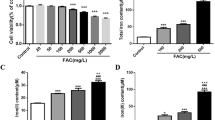
The oligodendroglial cell line OLN-93 was used as model system to investigate the consequences of iron deprivation or iron excess on cell proliferation. Presence of ferric or ferrous iron chelators inhibited the proliferation of OLN-93 cells in a time and concentration dependent manner, while the application of a molar excess of ferric ammonium citrate (FAC) prevented the inhibition of proliferation by the chelator deferoxamine. Proliferation of OLN-93 cells was not affected by incubation with 300 μM iron that was applied in the form of FAC, FeCl2, ferrous ammonium sulfate or iron oxide nanoparticles, although the cells efficiently accumulated iron during exposure to each of these iron sources. The highest specific iron content was observed for cells that were exposed to the nanoparticles. These data demonstrate that the proliferation of OLN-93 cells depends strongly on the availability of iron and that these cells efficiently accumulate iron from various extracellular iron sources.








Similar content being viewed by others
References
Galaris D, Skiada V, Barbouti A (2008) Redox signaling and cancer: the role of “labile” iron. Cancer Lett 266:21–29
Kruszewski M (2003) Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res 531:81–92
Dringen R, Bishop GM, Koeppe M et al (2007) The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res 32:1884–1890
Brodie C, Siriwardana G, Lucas J et al (1993) Neuroblastoma sensitivity to growth inhibition by deferrioxamine: evidence for a block in G1 phase of the cell cycle. Cancer Res 53:3968–3975
Lederman HM, Cohen A, Lee JW et al (1984) Deferoxamine: a reversible S-phase inhibitor of human lymphocyte proliferation. Blood 64:748–753
Green DA, Antholine WE, Wong SJ et al (2001) Inhibition of malignant cell growth by 311, a novel iron chelator of the pyridoxal isonicotinoyl hydrazone class: effect on the R2 subunit of ribonucleotide reductase. Clin Cancer Res 7:3574–3579
Cooper CE, Lynagh GR, Hoyes KP et al (1996) The relationship of intracellular iron chelation to the inhibition and regeneration of human ribonucleotide reductase. J Biol Chem. 271:20291–20299
Thelander L, Graslund A, Thelander M (1983) Continual presence of oxygen and iron required for mammalian ribonucleotide reduction: possible regulation mechanism. Biochem Biophys Res Commun 110:859–865
Nyholm S, Mann GJ, Johansson AG et al (1993) Role of ribonucleotide reductase in inhibition of mammalian cell growth by potent iron chelators. J Biol Chem. 268:26200–26205
Dayani PN, Bishop MC, Black K et al (2004) Desferoxamine (DFO)-mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J Neurooncol 67:367–377
Richardson DR (1997) Potential of iron chelators as effective antiproliferative agents. Can J Physiol Pharmacol 75:1164–1180
Yu Y, Kovacevic Z, Richardson DR (2007) Tuning cell cycle regulation with an iron key. Cell Cycle. 6:1982–1994
Connor JR, Menzies SL (1996) Relationship of iron to oligodendrocytes and myelination. Glia. 17:83–93
Benkovic SA, Connor JR (1993) Ferritin, transferrin, and iron in selected regions of the adult and aged rat brain. J Comp Neurol 338:97–113
Garrick MD, Garrick LM (2009) Cellular iron transport. Biochim Biophys Acta 1790:309–325
Hulet SW, Menzies S, Connor JR (2002) Ferritin binding in the developing mouse brain follows a pattern similar to myelination and is unaffected by the jimpy mutation. Dev Neurosci 24:208–213
Todorich B, Pasquini JM, Garcia CI et al (2009) Oligodendrocytes and myelination: the role of iron. Glia. 57:467–478
Song N, Jiang H, Wang J et al (2007) Divalent metal transporter 1 up-regulation is involved in the 6-hydroxydopamine-induced ferrous iron influx. J Neurosci Res 35:3118–3126
Burdo JR, Menzies SL, Simpson IA et al (2001) Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res 66:1198–1207
Todorich B, Zhang X, Slagle-Webb B et al (2008) Tim-2 is the receptor for H-ferritin on oligodendrocytes. J Neurochem 107:1495–1505
Hulet SW, Hess EJ, Debinski W et al (1999) Characterization and distribution of ferritin binding sites in the adult mouse brain. J Neurochem 72:868–874
Richter-Landsberg C, Heinrich M (1996) OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J Neurosci Res 45:161–173
Geppert M, Hohnholt M, Gaetjen L et al (2009) Accumulation of iron oxide nanoparticles by cultured brain astrocytes. J Biomed Nanotechnol. 5:285–293
Riemer J, Hoepken HH, Czerwinska H et al (2004) Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem 331:370–375
Bishop GM, Robinson SR (2001) Quantitative analysis of cell death and ferritin expression in response to cortical iron: implications for hypoxia-ischemia and stroke. Brain Res 907:175–187
Moos T, Mollgard K (1993) A sensitive post-DAB enhancement technique for demonstration of iron in the central nervous system. Histochemistry. 99:471–475
Dringen R, Kussmaul L, Hamprecht B (1998) Detoxification of exogenous hydrogen peroxide and organic hydroperoxides by cultured astroglial cells assessed by microtiter plate assay. Brain Res Brain Res Protoc. 2:223–228
Schmidt MM, Dringen R (2009) Differential effects of iodoacetamide and iodoacetate on glycolysis and glutathione metabolism of cultured astrocytes. Front Neuroenergetics. 1:1–10
Lowry OH, Rosebrough NJ, Farr AL et al (1951) Protein measurement with the Folin phenol reagent. J Biol Chem. 193:265–275
Hoepken HH, Korten T, Robinson SR et al (2004) Iron accumulation, iron-mediated toxicity and altered levels of ferritin and transferrin receptor in cultured astrocytes during incubation with ferric ammonium citrate. J Neurochem 88:1194–1202
Gharagozloo M, Khoshdel Z, Amirghofran Z (2008) The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: a comparison with desferrioxamine. Eur J Pharmacol 589:1–7
Keberle H (1964) The biochemistry of desferrioxamine and its relation to iron metabolism. Ann N Y Acad Sci 119:758–768
Richardson DR, Baker E (1994) Two saturable mechanisms of iron uptake from transferrin in human melanoma cells: the effect of transferrin concentration, chelators, and metabolic probes on transferrin and iron uptake. J Cell Physiol 161:160–168
Ware JL, Paulson DF, Webb KS (1984) 1, 10-Phenanthroline reversibility inhibits proliferation of two human prostate carcinoma cell lines (PC-3 and DU145). Biochem Biophys Res Commun 124:538–543
Szuts D, Krude T (2004) Cell cycle arrest at the initiation step of human chromosomal DNA replication causes DNA damage. J Cell Sci 117:4897–4908
Trinder D, Morgan E (1998) Mechanisms of ferric citrate uptake by human hepatoma cells. Am J Physiol 275:G279–G286
Zhu L, Glahn RP, Yeung CK et al (2006) Iron uptake by Caco-2 cells from NaFeEDTA and FeSO4: Effects of ascorbic acid, pH, and a Fe(II) chelating agent. J Agric Food Chem 54:7924–7928
Liddell JR, Hoepken HH, Crack PJ et al (2006) Glutathione peroxidase 1 and glutathione are required to protect mouse astrocytes from iron-mediated hydrogen peroxide toxicity. J Neurosci Res 84:578–586
Brand A, Schonfeld E, Isharel I et al (2008) Docosahexaenoic acid-dependent iron accumulation in oligodendroglia cells protects from hydrogen peroxide-induced damage. J Neurochem 105:1325–1335
Schroder I, Johnson E, de Vries S (2003) Microbial ferric iron reductases. FEMS Microbiol Rev 27:427–447
Tulpule K, Robinson SR, Bishop GM et al (2010) Uptake of ferrous iron by cultured rat astrocytes. J Neurosci Res 88:563–571
Attieh ZK, Mukhopadhyay CK, Seshadri V et al (1999) Ceruloplasmin ferroxidase activity stimulates cellular iron uptake by a trivalent cation-specific transport mechanism. J Biol Chem. 274:1116–1123
Conrad ME, Umbreit JN, Moore EG et al (2000) Separate pathways for cellular uptake of ferric and ferrous iron. Am J Physiol Gastrointest Liver Physiol. 279:G767–G774
Schonberg DL, McTigue DM (2009) Iron is essential for oligodendrocyte genesis following intraspinal macrophage activation. Exp Neurol 218:64–74
Islam T, Josephson L (2009) Current state and future applications of active targeting in malignancies using superparamagnetic iron oxide nanoparticles. Cancer Biomark. 5:99–107
Ge Y, Zhang Y, Xia J et al (2009) Effect of surface charge and agglomerate degree of magnetic iron oxide nanoparticles on KB cellular uptake in vitro. Colloids Surf B Biointerfaces. 73:294–301
Weinstein JS, Varallyay CG, Dosa E et al (2010) Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab 30:15–35
Xie J, Wang J, Niu G et al (2010) Human serum albumin coated iron oxide nanoparticles for efficient cell labeling. Chem Commun (Camb). 46:433–435
Pisanic TR 2nd, Blackwell JD, Shubayev VI et al (2007) Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28:2572–2581
Dunning MD, Lakatos A, Loizou L et al (2004) Superparamagnetic iron oxide-labeled Schwann cells and olfactory ensheathing cells can be traced in vivo by magnetic resonance imaging and retain functional properties after transplantation into the CNS. J Neurosci 24:9799–9810
Falangola MF, Lee SP, Nixon RA et al (2005) Histological co-localization of iron in Abeta plaques of PS/APP transgenic mice. Neurochem Res 30:201–205
Acknowledgments
Michaela Hohnholt and Mark Geppert are members of the Ph.D. graduate school nanoToxCom at the University of Bremen. Michaela Hohnholt is financially supported by a grant from the University Bremen (BFK) and Mark Geppert is a recipient of a Ph.D. fellowship from the Hans-Böckler Stiftung. We like to thank Prof. C. Richter-Landsberg (Oldenburg, Germany) for kindly providing us with OLN-93 cells and Maike Schmidt for her help with the microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hohnholt, M., Geppert, M. & Dringen, R. Effects of Iron Chelators, Iron Salts, and Iron Oxide Nanoparticles on the Proliferation and the Iron Content of Oligodendroglial OLN-93 Cells. Neurochem Res 35, 1259–1268 (2010). https://doi.org/10.1007/s11064-010-0184-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-010-0184-5