Abstract
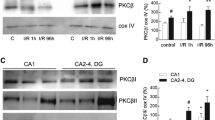
Here we report effect of ischemia-reperfusion on mitochondrial Ca2+ uptake and activity of complexes I and IV in rat hippocampus. By performing 4-vessel occlusion model of global brain ischemia, we observed that 15 min ischemia led to significant decrease of mitochondrial capacity to accumulate Ca2+ to 80.8% of control whereas rate of Ca2+ uptake was not significantly changed. Reperfusion did not significantly change mitochondrial Ca2+ transport. Ischemia induced progressive inhibition of complex I, affecting final electron transfer to decylubiquinone. Minimal activity of complex I was observed 24 h after ischemia (63% of control). Inhibition of complex IV activity to 80.6% of control was observed 1 h after ischemia. To explain the discrepancy between impact of ischemia on rate of Ca2+ uptake and activities of both complexes, we performed titration experiments to study relationship between inhibition of particular complex and generation of mitochondrial transmembrane potential (ΔΨm). Generation of a threshold curves showed that complex I and IV activities must be decreased by approximately 40, and 60%, respectively, before significant decline in ΔΨm was documented. Thus, mitochondrial Ca2+ uptake was not significantly affected by ischemia-reperfusion, apparently due to excess capacity of the complexes I and IV. Inhibition of complex I is favourable of reactive oxygen species (ROS) generation. Maximal oxidative modification of membrane proteins was documented 1 h after ischemia. Although enhanced formation of ROS might contribute to neuronal injury, depressed activities of complex I and IV together with unaltered rate of Ca2+ uptake are conditions favourable of initiation of other cell degenerative pathways like opening of mitochondrial permeability transition pore or apoptosis initiation, and might represent important mechanism of ischemic damage to neurones.





Similar content being viewed by others
References
Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG (2006) Neuronal-glial glucose oxidation and glutamatergic–GABAergic function. J Cereb Blood Flow Metab 26:865–877. doi:10.1038/sj.jcbfm.9600263
Yuan J, Yanker BA (2000) Apoptosis in the nervous system. Nature 407:802–809. doi:10.1038/35037739
Duchen MR (2000) Mitochondria and Ca(2 +) in cell physiology and pathophysiology. Cell Calcium 28:339–348. doi:10.1054/ceca.2000.0170
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4:517–529. doi:10.1038/nrm1155
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1:11–21. doi:10.1038/35036035
Rizzuto R (2001) Intracellular Ca(2 +) pools in neuronal signalling. Curr Opin Neurobiol 11:306–311. doi:10.1016/S0959-4388(00)00212-9
Tang Y, Zucker RS (1997) Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 18:483–491. doi:10.1016/S0896-6273(00)81248-9
Montero M, Alonso MT, Carnicero E, Cuchillo-Ibanez I, Albillos A, Garcia AG, Garcia-Sancho J, Alvarez J (2000) Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2 + transients that modulate secretion. Nat Cell Biol 2:57–61. doi:10.1038/35000001
Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 287:C817–C833. doi:10.1152/ajpcell.00139.2004
Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD (2004) Calcium and mitochondria. FEBS Lett 567:96–102. doi:10.1016/j.febslet.2004.03.071
Budd SL (1998) Mechanisms of neuronal damage in brain hypoxia/ischemia: focus on the role of mitochondrial calcium accumulation. Pharmacol Ther 80:203–229. doi:10.1016/S0163-7258(98)00029-1
Murphy AN, Fiskum G, Beal MF (1999) Mitochondria in neurodegeneration: bioenergetic function in cell life and death. J Cereb Blood Flow Metab 19:231–245. doi:10.1097/00004647-199903000-00001
Duchen MR (2004) Mitochondria in health and disease: perspectives on a new mitochondrial biology. Mol Aspects Med 25:365–451
Nicholls DG, Johnson-Cadwell L, Vesce S, Jekabsons M, Yadava N (2007) Bioenergetics of mitochondria in cultured neurons and their role in glutamate excitotoxicity. J Neurosci Res 85:3206–3212. doi:10.1002/jnr.21290
Sattler R, Tymianski M (2000) Molecular mechanisms of calcium-dependent excitotoxicity. J Mol Med 78:3–13. doi:10.1007/s001090000077
Bano D, Nicotera P (2007) Ca2+signals and neuronal death in brain ischemia. Stroke 38:674–676. doi:10.1161/01.STR.0000256294.46009.29
Bevers MB, Neumar RW (2008) Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab 28:655–673. doi:10.1038/sj.jcbfm.9600595
White RJ, Reynolds IJ (1995) Mitochondria and Na+/CaCa2+exchange buffer glutamate-induced calcium loads in cultured cortical neurons. J Neurosci 15:1318–1328
Wang GJ, Thayer SA (1996) Sequestration of glutamate-induced CaCa2+loads by mitochondria in cultured rat hippocampal neurons. J Neurophysiol 76:1611–1621
White RJ, Reynolds IJ (1996) Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J Neurosci 16:5688–5697
Khodorov B, Pinelis V, Vergun O, Storozhevykh T, Vinskaya N (1996) Mitochondrial deenergization underlies neuronal calcium overload following a prolonged glutamate challenge. FEBS Lett 397:230–234. doi:10.1016/S0014-5793(96)01139-8
Schinder AF, Olson EC, Spitzer NC, Montal M (1996) Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci 16:6125–6133
Vergun O, Keelan J, Khodorov BI, Duchen MR (1999) Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J Physiol 519:451–466. doi:10.1111/j.1469-7793.1999.0451m.x
Peng TI, Jou MJ, Sheu SS, Greenamyre JT (1998) Visualization of NMDA receptor-induced mitochondrial calcium accumulation in striatal neurons. Exp Neurol 149:1–12. doi:10.1006/exnr.1997.6599
Budd SL, Nicholls DG (1996) Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem 67:2282–2291
Stout AK, Raphael HM, Kanterewicz BI, Klann E, Reynolds IJ (1998) Glutamate-induced neuron death requires mitochondrial calcium uptake. Nat Neurosci 1:366–373. doi:10.1038/1577
Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF, Melcher T, Gonzalez-Zulueta M, Nikolich K, Wieloch T (2003) Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med 9:1062–1068. doi:10.1038/nm903
Pivovarova NB, Nguyen HV, Winters CA, Brantner CA, Smith CL, Andrews SB (2004) Excitotoxic calcium overload in a subpopulation of mitochondria triggers delayed death in hippocampal neurons. J Neurosci 24:5611–5622. doi:10.1523/JNEUROSCI.0531-04.2004
Ward MW, Huber HJ, Weisová P, Düssmann H, Nicholls DG, Prehn JH (2007) Mitochondrial and plasma membrane potential of cultured cerebellar neurons during glutamate-induced necrosis, apoptosis, and tolerance. J Neurosci 27:8238–8249. doi:10.1523/JNEUROSCI.1984-07.2007
Allen KL, Almeida A, Bates TE, Clark JB (1995) Changes of respiratory chain activity in mitochondrial and synaptosomal fractions isolated from the gerbil brain after graded ischaemia. J Neurochem 64:2222–2229
Almeida A, Allen KL, Bates TE, Clark JB (1995) Effect of reperfusion following cerebral ischaemia on the activity of the mitochondrial respiratory chain in the gerbil brain. J Neurochem 65:1698–1703
Fiskum G, Murphy AN, Beal MF (1999) Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab 19:351–369. doi:10.1097/00004647-199904000-00001
Zaidan E, Sims NR (1994) The calcium content of mitochondria from brain subregions following short-term forebrain ischemia and recirculation in the rat. J Neurochem 63:1812–1819
Sciamanna MA, Zinkel J, Fabi AY, Lee CP (1992) Ischemic injury to rat forebrain mitochondria and cellular calcium homeostasis. Biochim Biophys Acta 1134:223–232. doi:10.1016/0167-4889(92)90180-J
Sciamanna MA, Lee CP (1993) Ischemia/reperfusion-induced injury of forebrain mitochondria and protection by ascorbate. Arch Biochem Biophys 305:215–224. doi:10.1006/abbi.1993.1414
Racay P, Tatarkova Z, Drgova A, Kaplan P, Dobrota D (2007) Effect of ischemic preconditioning on mitochondrial dysfunction and mitochondrial p53 translocation after transient global cerebral ischemia in rats. Neurochem Res 32:1823–1832. doi:10.1007/s11064-007-9437-3
Polosa PL, Attardi G (1991) Distinctive pattern and translational control of mitochondrial protein synthesis in rat brain synaptic endings. J Biol Chem 266:10011–10017
Racay P (2008) Effect of magnesium on calcium-induced depolarisation of mitochondrial transmembrane potential. Cell Biol Int 32:136–145. doi:10.1016/j.cellbi.2007.08.024
Bernardi P (1999) Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev 79:1127–1155
Friberg H, Wieloch T, Castilho RF (2002) Mitochondrial oxidative stress after global brain ischemia in rats. Neurosci Lett 334:111–114. doi:10.1016/S0304-3940(02)01116-3
Racay P, Kaplán P, Lehotský J (2000) Ischemia-induced inhibition of active calcium transport into gerbil brain microsomes: effect of anesthetics and models of ischemia. Neurochem Res 25:285–292. doi:10.1023/A:1007587907047
Kitano H, Kirsch JR, Hurn PD, Murphy SJ (2007) Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab 27:1108–1128. doi:10.1038/sj.jcbfm.9600410
Davey GP, Peuchen S, Clark JB (1998) Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem 273:12753–12757. doi:10.1074/jbc.273.21.12753
Folbergrová J, Jesina P, Drahota Z, Lisý V, Haugvicová R, Vojtísková A, Houstĕk J (2007) Mitochondrial complex I inhibition in cerebral cortex of immature rats following homocysteic acid-induced seizures. Exp Neurol 204:597–609. doi:10.1016/j.expneurol.2006.12.010
Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, Rossignol R (2007) Mitochondrial bioenergetics and structural network organization. J Cell Sci 120:838–848. doi:10.1242/jcs.03381
Grivennikova VG, Kapustin AN, Vinogradov AD (2001) Catalytic activity of NADH-ubiquinone oxidoreductase (complex I) in intact mitochondria. Evidence for the slow active/inactive transition. J Biol Chem 276:9038–9044. doi:10.1074/jbc.M009661200
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443:787–795. doi:10.1038/nature05292
Chan PH (2001) Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21:2–14. doi:10.1097/00004647-200101000-00002
Sousa SC, Maciel EN, Vercesi AE, Castilho RF (2003) Ca2+-induced oxidative stress in brain mitochondria treated with the respiratory chain inhibitor rotenone. FEBS Lett 543:179–183. doi:10.1016/S0014-5793(03)00421-6
Panov A, Dikalov S, Shalbuyeva N, Taylor G, Sherer T, Greenamyre JT (2005) Rotenone model of Parkinson disease: multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J Biol Chem 280:42026–42035. doi:10.1074/jbc.M508628200
Yadava N, Nicholls DG (2007) Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci 27:7310–7317. doi:10.1523/JNEUROSCI.0212-07.2007
Perier C, Bové J, Wu DC, Dehay B, Choi DK, Jackson-Lewis V, Rathke-Hartlieb S, Bouillet P, Strasser A, Schulz JB, Przedborski S, Vila M (2007) Two molecular pathways initiate mitochondria-dependent dopaminergic neurodegeneration in experimental Parkinson’s disease. Proc Natl Acad Sci USA 104:8161–8166. doi:10.1073/pnas.0609874104
Duan Y, Gross RA, Sheu SS (2007) Ca2+-dependent generation of mitochondrial reactive oxygen species serves as a signal for poly(ADP-ribose) polymerase-1 activation during glutamate excitotoxicity. J Physiol 585:741–758. doi:10.1113/jphysiol.2007.145409
Racay P, Tatarkova Z, Drgova A, Kaplan P, Dobrota D (2007) Effect of ischemic preconditioning on ischemia-induced mitochondrial dysfunction and protein modification. J Neurochem 101(Suppl. 1):44–45
Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ (2005) Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc Natl Acad Sci USA 102:12005–12010. doi:10.1073/pnas.0505294102
Tanaka H, Yokota H, Jover T, Cappuccio I, Calderone A, Simionescu M, Bennett MV, Zukin RS (2004) Ischemic preconditioning: neuronal survival in the face of caspase-3 activation. J Neurosci 24:2750–2759. doi:10.1523/JNEUROSCI.5475-03.2004
Acknowledgments
This work was supported by the Ministry of Education of Slovak Republic (grants VEGA 1/4255/07 and VVCE 0064-07). Authors are grateful to Zdenka Cetlova for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Racay, P., Tatarkova, Z., Chomova, M. et al. Mitochondrial Calcium Transport and Mitochondrial Dysfunction After Global Brain Ischemia in Rat Hippocampus. Neurochem Res 34, 1469–1478 (2009). https://doi.org/10.1007/s11064-009-9934-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-009-9934-7