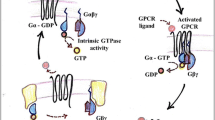
Receptors of the 7-TMR group (G protein-coupled receptors, GPCRs) are uniquely important from the fundamental physiological aspect because they mediate the effects of a majority of the known neuromediators and hormones. According to a widely accepted paradigm, activation of heterotrimetric G proteins is the initial step in the process of transduction of signals from these receptors to effectors. In recent times, increasing proof of the existence of G protein-independent mechanisms providing initiation of biochemical signals by these receptors and modulation of the excitability of the neurons has been accumulated. According to these data, 7-TMRs are able to interact with a few cytoplasmic scaffold proteins and take part in various protein-to-protein interactions. Despite the fact that functional importance of many such interactions remains unknown at present, the respective results indicate that the above processes are capable not only of providing G protein-independent transmission of signals from the 7-TMRs, but also of influencing pharmacological characteristics, cellular localization, and compartmentalization of the mentioned receptors.
Similar content being viewed by others
References
R. J. Lefkowitz, “Historical review: a brief history and personal retrospective of seven-transmembrane receptors,” Trends Pharmacol. Sci., 25, 413-422 (2004).
D. K. Vassilatis, J. G. Hohmann, H. Zeng, et al., “The G protein-coupled receptor repertoires of human and mouse,” Proc. Natl. Acad. Sci. USA, 100, 4903-4908 (2003).
J. Bockaert and J. P. Pin, “Molecular tinkering of G protein-coupled receptors: an evolutionary success,” EMBO J., 18, 1723-1729 (1999).
D. Filmore, “It’s a GPCR world,” Mod. Drug Discov. (Am. Chem. Soc.), 7, Issue 11, 24-28 (2004).
J. P. Overington, B. Al-Lazikani, and A. L. Hopkins, “How many drug targets are there?” Natl. Rev. Drug Discov., 5, 993-996 (2006).
A. Wise, K. Gearing, and S. Rees, “Target validation of G-protein coupled receptors,” Drug Discov. Today, 7, 235-246 (2002).
J. M. Baldwin, “The probable arrangement of the helices in G protein-coupled receptors,” EMBO J., 12, 1693- 1703 (1993).
D. Spengler, C. Waeber, C. Pantaloni, et al., “Differential signal transduction by five splice variants of the PACAP receptor,” Nature, 365, 170-175 (1993).
J. P. Pin and J. Bockaert, “Get receptive to metabotropic glutamate receptors,” Curr. Opin. Neurobiol., 5, 342-349 (1995).
J. Wess, “G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition,” FASEB J., 11, 346-354 (1997).
U. Gether, “Uncovering molecular mechanisms involved in activation of G protein-coupled receptors,” Endocrinol. Rev., 21, 90-113 (2000).
R. Neuhaus, B. F. Reber, and H. Reuter, “Regulation of bradykinin- and ATP-activated Ca2+-permeable channels in rat pheochromocytoma (PC12) cells,” J. Neurosci., 11, 3984-3990 (1991).
K. Koyano, B. M. Velimirovic, J. J. Grigg, et al., “Two signal transduction mechanisms of substance P-induced depolarization in locus coeruleus neurons,” Eur. J. Neurosci., 5, 1189-1197 (1993).
S. A. Thomas and R. I. Hume, “Single potassium channel currents activated by extracellular ATP in developing chick skeletal muscle: a role for second messengers,” J. Neurophysiol., 69, 1556-1566 (1993).
W. A. Twitchell and S. G. Rane, “Nucleotide-independent modulation of Ca2+-dependent K+ channel current by a μ-type opioid receptor," Mol. Pharmacol., 46, 793-798 (1994).
J. Kehoe, “Glutamate activates a K+ conductance increase in Aplysia neurons that appears to be independent of G proteins,” Neuron, 13, 691-702 (1994).
N. C. Guerineau, J. L. Bossu, B. H. Gahwiler, et al., “Activation of a nonselective cationic conductance by metabotropic glutamatergic and muscarinic agonists in CA3 pyramidal neurons of the rat hippocampus,” J. Neurosci., 15, 4395-4407 (1995).
F. Zheng, H. Hasuo, and J. P. Gallagher, “1S,3R-ACPDpreferring inward current in rat dorsolateral septal neurons is mediated by a novel excitatory amino acid receptor,” Neuropharmacology, 34, 905-917 (1995).
F. A. Abdulla and P. A. Smith, “Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism,” J. Neurosci., 17, 8721-8728 (1997).
C. Heuss, M. Scanziani, B. H. Gahwiler, et al., “G-protein-independent signaling mediated by metabotropic glutamate receptors,” Nat. Neurosci., 2, 1070-1077 (1999).
Q. Q. Sun and N. Dale, “G-proteins are involved in 5-HT receptor-mediated modulation of N- and P/Q- but not T-type Ca2+ channels,” J. Neurosci., 19, 890-899 (1999).
O. Iegorova, A. Fisyunov and O. Krishtal, “G-proteinindependent modulation of P-type calcium channels by μ-opioids in Purkinje neurons of rat," Neurosci. Lett., 480, 106-111 (2010).
J. Chevesich, A. J. Kreuz, and C. Montell, “Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex,” Neuron, 18, 95-105 (1997).
X. Z. Xu, A. Choudhury, X. Li, and C. Montell, “Coordination of an array of signaling proteins through homo- and heteromeric interactions between PDZ domains and target proteins,” J. Cell Biol., 142, 545-555 (1998).
L. A. Hu, Y. Tang, W. E. Miller, et al., “β1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of β-1-adrenergic receptor interaction with N-methyl-D-aspartate receptors,” J. Biol. Chem., 275, 38659-38666 (2000).
R. A. Hall, R. T. Premont, C. W. Chow, et al., “The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange,” Nature, 392, 626-630 (1998).
C. Becamel, A. Figge, S. Poliak, et al., “Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1,” J. Biol. Chem., 276, 12974-12982 (2001).
J. C. Tu, B. Xiao, S. Naisbitt, et al., “Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins,” Neuron, 23, 583-592 (1999).
J. Kitano, K. Kimura, Y. Yamazaki, et al., “Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins,” J. Neurosci., 22, 1280-1289 (2002).
M. Shih, F. Lin, J. D. Scott, et al., “Dynamic complexes of β2-adrenergic receptors with protein kinases and phosphatases and the role of gravin," J. Biol. Chem., 274, 1588-1595 (1999).
I. D. Fraser, M. Cong, J. Kim, et al., “Assembly of an A kinase-anchoring protein-β(2)-adrenergic receptor complex facilitates receptor phosphorylation and signaling," Current Biol., 10, 409-412 (2000).
A. Kato, F. Ozawa, Y. Saitoh, et al., “Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors,” J. Biol. Chem., 273, 23969-23975 (1998).
B. Xiao, J. C. Tu, R. S. Petralia, et al., “Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins,” Neuron, 21, 707-716 (1998).
M. S. Ali, P. P. Sayeski, L. B. Dirksen, et al., “Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor,” J. Biol. Chem., 272, 23382-23388 (1997).
J. H. White, R. A. McIllhinney, A. Wise, et al., “The GABAB receptor interacts directly with the related transcription factors CREB2 and ATFx,” Proc. Natl. Acad. Sci. USA, 97, 13967-13972 (2000).
R. B. Nehring, H. P. Horikawa, F. O. El, et al., “The metabotropic GABAB receptor directly interacts with the activating transcription factor 4,” J. Biol. Chem., 275, 35185-35191 (2000).
Y. Tang, L. A. Hu, W. E. Miller, et al., “Identification of the endophilins (SH3p4/p8/p13) as novel binding partners for the β1-adrenergic receptor," Proc. Natl. Acad. Sci. USA, 96, 12559-12564 (1999).
W. Cao, L. M. Luttrell, A. V. Medvedev, et al., “Direct binding of activated c-Src to the β3-adrenergic receptor is required for MAP kinase activation," J. Biol. Chem., 275, 38131-38134 (2000).
F. Liu, Q. Wan, Z. B. Pristupa, et al., “Direct protein-protein coupling enables cross-talk between dopamine D5 and γ-aminobutyric acid A receptors," Nature, 403, 274-280 (2000).
E. Hawrot, Y. Xiao, Q. L. Shi, et al., “Demonstration of a tandem pair of complement protein modules in GABAB receptor 1a,” FEBS Lett., 432, 103-108 (1998).
M. Stacey, H. H. Lin, S. Gordon, and A. J. McKnight, “LNB-TM7, a group of seven-transmembrane proteins related to family-B G-protein-coupled receptors,” Trends Biochem. Sci., 25, 284-289 (2000).
M. Sheng and C. Sala, “PDZ domains and the organization of supramolecular complexes,” Annu. Rev. Neurosci., 24, 1-29 (2001).
K. Scott and C. S. Zuker, “Assembly of the Drosophila phototransduction cascade into a signaling complex shapes elementary responses,” Nature, 395, 805-808 (1998).
P. W. Gean, C. C. Huang, J. H. Lin, and J. J. Tsai, “Sustained enhancement of NMDA receptor-mediated synaptic potential by isoproterenol in rat amygdalar slices,” Brain Res., 594, 331-334 (1992).
C. C. Huang, J. J. Tsai, and P. W. Gean, “Enhancement of NMDA receptor-mediated synaptic potential by isoproterenol is blocked by Rp-adenosine 3’,5’-cyclic monophosphothioate,” Neurosci. Lett., 161, 207-210 (1993).
I. M. Raman, G. Tong, and C. E. Jahr, “β-adrenergic regulation of synaptic NMDA receptors by cAMPdependent protein kinase," Neuron, 16, 415-421 (1996).
R. A. Hall, L. S. Ostedgaard, R. T. Premont, et al., “A C terminal motif found in the β2-adrenergic receptor, P2Y1 receptor and cystic fibrosis transmembrane conductance regulator determines binding to the Na+/H+ exchanger regulatory factor family of PDZ proteins,” Proc. Natl. Acad. Sci. USA, 95, 8496-8501 (1998).
E. J. Weinman, C. Minkoff, and S. Shenolikar, “Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA,” Am. J. Physiol. (Renal Physiol.), 279, F393-F399 (2000).
T. T. Cao, H. W. Deacon, D. Reczek, et al., “A kinaseregulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor," Nature, 401, 286-290 (1999).
A. J. McLean and G. Milligan, “Ligand regulation of green fluorescent protein-tagged forms of the human β(1)- and β(2) adrenoreceptors; comparisons with the unmodified receptors," Br. J. Pharmacol., 130, 1825-1832 (2000).
L. A. Hu, W. Chen, R. T. Premont, et al., “G proteincoupled receptor kinase 5 regulates β1-adrenergic receptor association with PSD-95," J. Biol. Chem., 277, 1607-1613 (2002).
C. Ullmer, K. Schmuck, A. Figge, and H. Lubbert, “Cloning and characterization of MUPP1, a novel PDZ domain protein,” FEBS Lett., 424, 63-68 (1998).
H. Boudin, A. Doan, J. Xia, et al., “Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site,” Neuron, 28, 485-497 (2000).
K. K. Dev, Y. Nakajima, J. Kitano, et al., “PICK1 interacts with and regulates PKC phosphorylation of mGLUR7,” J. Neurosci., 20, 7252-7257 (2000).
F. O. El, J. Airas, E. Wischmeyer, et al., “Interaction of the C terminal tail region of the metabotropic glutamate receptor 7 with the protein kinase C substrate PICK1,” Eur. J. Neurosci., 12, 4215-4221 (2000).
H. Boudin and A. M. Craig, “Molecular determinants for PICK1 synaptic aggregation and mGluR7a receptor coclustering: role of the PDZ, coiled-coil, and acidic domains,” J. Biol. Chem., 276, 30270-30276 (2001).
S. Lim, S. Naisbitt, J. Yoon, et al., “Characterization of the Shank family of synaptic proteins. Multiple genes, alternative splicing, and differential expression in brain and development,” J. Biol. Chem., 274, 29510-29518 (1999).
M. Sheng and E. Kim, “The Shank family of scaffold proteins,” J. Cell Sci., 113, Part 11, 1851-1856 (2000).
M. Colledge and J. D. Scott, “AKAPs: from structure to function,” Trends Cell Biol., 9, 216-221 (1999).
G. Fan, E. Shumay, H. Wang, and C. C. Malbon, “The scaffold protein gravin (cAMP-dependent protein kinaseanchoring protein 250) binds the β2-adrenergic receptor via the receptor cytoplasmic Arg-329 to Leu-413 domain and provides a mobile scaffold during desensitization," J. Biol. Chem., 276, 24005-24014 (2001).
F. Lin, H. Wang, and C. C. Malbon, “Gravin-mediated formation of signaling complexes in β2-adrenergic receptor desensitization and resensitization," J. Biol. Chem., 275, 19025-19034 (2000).
M. Cong, S. J. Perry, F. T. Lin, et al., “Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79,” J. Biol. Chem., 276, 15192-15199 (2001).
P. R. Brakeman, A. A. Lanahan, R. O’Brien, et al., “Homer: a protein that selectively binds metabotropic glutamate receptors,” Nature, 386, 284-288 (1997).
J. C. Tu, B. Xiao, J. P. Yuan, et al., “Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors,” Neuron, 21, 717-726 (1998).
F. Ciruela, M. M. Soloviev, and R. A. McIlhinney, “Co-expression of metabotropic glutamate receptor type 1α with Homer-1a/Vesl-1S increases the cell surface expression of the receptor," Biochem. J., 341, Part 3, 795-803 (1999).
K. W. Roche, J. C. Tu, R. S. Petralia, et al., “Homer 1b regulates the trafficking of group I metabotropic glutamate receptors,” J. Biol. Chem., 274, 25953-25957 (1999).
F. Ango, J. P. Pin, J. C. Tu, et al., “Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by Homer1 proteins and neuronal excitation,” J. Neurosci., 20, 8710-8716 (2000).
F. Ciruela, M. M. Soloviev, W. Y. Chan, and R. A. McIlhinney, “Homer-1c/Vesl-1L modulates the cell surface targeting of metabotropic glutamate receptor type 1α: evidence for an anchoring function," Mol. Cell Neurosci., 15, 36-50 (2000).
P. J. Kammermeier, B. Xiao, J. C. Tu, et al., “Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels,” J. Neurosci., 20, 7238-7245 (2000).
F. Ango, L. Prezeau, T. Muller, et al., “Agonistindependent activation of metabotropic glutamate receptors by the intracellular protein Homer,” Nature, 411, 962-965 (2001).
V. Coutinho, I. Kavanagh, H. Sugiyama, et al., “Characterization of a metabotropic glutamate receptor type 5-green fluorescent protein chimera (mGluR5-GFP): pharmacology, surface expression, and differential effects of Homer-1a and Homer-1c,” Mol. Cell Neurosci., 18, 296-306 (2001).
R. Minakami, A. Kato, and H. Sugiyama, “Interaction of Vesl-1L/Homer 1c with syntaxin 13,” Biochem. Biophys. Res. Commun., 272, 466-471 (2000).
C. Schindler and J. E. Darnell, Jr., “Transcriptional responses to polypeptide ligands: the JAK-STAT pathway,” Annu. Rev. Biochem., 64, 621-651 (1995).
M. B. Marrero, V. J. Venema, H. Ju, et al., “Regulation of angiotensin II-induced JAK2 tyrosine phosphorylation: roles of SHP-1 and SHP-2,” Am. J. Physiol, 275, C1216-C1223 (1998).
M. S. Ali, P. P. Sayeski, and K. E. Bernstein, “Jak2 acts as both a STAT1 kinase and as a molecular bridge linking STAT1 to the angiotensin II AT1 receptor,” J. Biol. Chem., 275, 15586-15593 (2000).
P. P. Sayeski, M. S. Ali, S. J. Frank, and K. E. Bernstein, “The angiotensin II-dependent nuclear translocation of Stat1 is mediated by the Jak2 protein motif 231YRFRR,” J. Biol. Chem., 276, 10556-10563 (2001).
G. Hjalm, R. J. MacLeod, O. Kifor, et al., “Filamin-A binds to the carboxyl-terminal tail of the calciumsensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase,” J. Biol. Chem., 276, 34880-34887 (2001).
H. Awata, C. Huang, M. E. Handlogten, and R. T. Miller, “Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein,” J. Biol. Chem., 276, 34871-34879 (2001).
L. M. Luttrell, S. S. Ferguson, Y. Daaka, et al., “β arrestin-dependent formation of β2-adrenergic receptor- Src protein kinase complexes," Science, 283, 655-661 (1999).
S. Maudsley, K. L. Pierce, A. M. Zamah, et al., “The β(2)-adrenergic receptor mediates extracellular signal-regulated kinase activation via assembly of a multi-receptor complex with the epidermal growth factor receptor," J. Biol. Chem., 275, 9572-9580 (2000).
M. Li, J. C. Bermak, Z. W. Wang, and Q. Y. Zhou, “Modulation of dopamine D2 receptor signaling by actin-binding protein (ABP-280),” Mol. Pharmacol., 57, 446-452 (2000).
M. J. Lohse, J. L. Benovic, J. Codina, et al., “β arrestin: a protein that regulates β-adrenergic receptor function," Science, 248, 1547-1550 (1990).
H. Attramadal, J. L. Arriza, C. Aoki, et al., “β arrestin 2, a novel member of the arrestin/β arrestin gene family," J. Biol. Chem., 267, 17882-17890 (1992).
J. G. Krupnick and J. L. Benovic, “The role of receptor kinases and arrestins in G protein-coupled receptor regulation,” Annu. Rev. Pharmacol. Toxicol., 38, 289-319 (1998).
L. M. Luttrell, Y. Daaka, and R. J. Lefkowitz, “Regulation of tyrosine kinase cascades by G-proteincoupled receptors,” Curr. Opin. Cell Biol., 11, 177-183 (1999).
R. J. Lefkowitz, “G protein-coupled receptors. III. New roles for receptor kinases and β arrestins in receptor signaling and desensitization," J. Biol. Chem., 273, 18677-18680 (1998).
O. B. Goodman, Jr., J. G. Krupnick, F. Santini, et al., “β Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor,” Nature, 383, 447-450 (1996).
S. A. Laporte, R. H. Oakley, J. Zhang, et al., “The β2-adrenergic receptor/β arrestin complex recruits the clathrin adaptor AP-2 during endocytosis," Proc. Natl. Acad. Sci. USA, 96, 3712-3717 (1999).
S. A. Laporte, R. H. Oakley, J. A. Holt, et al., “The interaction of β arrestin with the AP-2 adaptor is required for the clustering of β2-adrenergic receptor into clathrin-coated pits," J. Biol. Chem., 275, 23120-23126 (2000).
P. H. McDonald, N. L. Cote, F. T. Lin, et al., “Identification of NSF as a β arrestin1-binding protein. Implications for β2-adrenergic receptor regulation," J. Biol. Chem., 274, 10677-10680 (1999).
A. Claing, W. Chen, W. E. Miller, et al., “β Arrestinmediated ADP-ribosylation factor 6 activation and β2-adrenergic receptor endocytosis,” J. Biol. Chem., 276, 42509-42513 (2001).
P. H. McDonald, C. W. Chow, W. E. Miller, et al., “β Arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3," Science, 290, 1574-1577 (2000).
W. E. Miller, P. H. McDonald, S. F. Cai, et al., “Identification of a motif in the carboxyl terminus of β arrestin 2 responsible for activation of JNK3,” J. Biol. Chem., 276, 27770-27777 (2001).
K. A. DeFea, J. Zalevsky, M. S. Thoma, et al., “β arrestindependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2,” J. Cell Biol., 148, 1267-1281 (2000).
L. M. Luttrell, F. L. Roudabush, E. W. Choy, et al., “Activation and targeting of extracellular signalregulated kinases by β arrestin scaffolds," Proc. Natl. Acad. Sci. USA, 98, 2449-2454 (2001).
W. E. Miller, S. Maudsley, S. Ahn, et al., “β Arrestin 1 interacts with the catalytic domain of the tyrosine kinase c-SRC. Role of β arrestin1-dependent targeting of c-SRC in receptor endocytosis," J. Biol. Chem., 275, 11312-11319 (2000).
S. R. Coughlin, “How the protease thrombin talks to cells,” Proc. Natl. Acad. Sci. USA, 96, 11023-11027 (1999).
K. Kaupmann, K. Huggel, J. Heid, et al., “Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors,” Nature, 386, 239-246 (1997).
D. Benke, M. Honer, C. Michel, et al., “γ-Aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution,” J. Biol. Chem., 274, 27323-27330 (1999).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fisyunov, А.I. Molecular Mechanisms of G Protein-Independent Signaling Mediated by 7-Transmembrane Receptors. Neurophysiology 44, 255–264 (2012). https://doi.org/10.1007/s11062-012-9295-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11062-012-9295-8