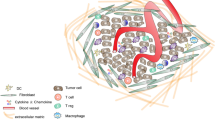
Abstract
Background
Focused ultrasound (FUS) is an emerging technology, offering the capability of tuning and prescribing thermal and mechanical treatments within the brain. While early works in utilizing this technology have mainly focused on maximizing the delivery of therapeutics across the blood–brain barrier (BBB), the potential therapeutic impact of FUS-induced controlled thermal and mechanical stress to modulate anti-tumor immunity is becoming increasingly recognized.
Objective
To better understand the roles of FUS-mediated thermal and mechanical stress in promoting anti-tumor immunity in central nervous system tumors, we performed a comprehensive literature review on focused ultrasound-mediated immunomodulation and immunotherapy in brain tumors.
Methods
First, we summarize the current clinical experience with immunotherapy. Then, we discuss the unique and distinct immunomodulatory effects of the FUS-mediated thermal and mechanical stress in the brain tumor-immune microenvironment. Finally, we highlight recent findings that indicate that its combination with immune adjuvants can promote robust responses in brain tumors.
Results
Along with the rapid advancement of FUS technologies into recent clinical trials, this technology through mild-hyperthermia, thermal ablation, mechanical perturbation mediated by microbubbles, and histotripsy each inducing distinct vascular and immunological effects, is offering the unique opportunity to improve immunotherapeutic trafficking and convert immunologically “cold” tumors into immunologically “hot” ones that are prone to generate prolonged anti-tumor immune responses.
Conclusions
While FUS technology is clearly accelerating concepts for new immunotherapeutic combinations, additional parallel efforts to detail rational therapeutic strategies supported by rigorous preclinical studies are still in need to leverage potential synergies of this technology with immune adjuvants. This work will accelerate the discovery and clinical implementation of new effective FUS immunotherapeutic combinations for brain tumor patients.

Similar content being viewed by others
Data Availability
All the associated data in this review paper are included in the paper (Tables) or can be found in the cited literature.
References
Meng Y, Hynynen K, Lipsman N (2021) Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol 17:7–22. https://doi.org/10.1038/s41582-020-00418-z
ter Haar >Gail, Coussios C, (2007) High intensity focused ultrasound: Physical principles and devices. Int J Hyperthermia 23:89–104. https://doi.org/10.1080/02656730601186138
ter Haar G (2007) Therapeutic applications of ultrasound. Prog Biophys Mol Biol 93:111–129
McDannold N, Clement GT, Black P et al (2010) Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: initial findings in 3 patients. Neurosurgery 66:323–332
Elias WJ, Huss D, Voss T et al (2013) A pilot study of focused ultrasound thalamotomy for essential tremor. N Engl J Med 369:640–648. https://doi.org/10.1056/NEJMoa1300962
Elias WJ, Lipsman N, Ondo WG et al (2016) A randomized trial of focused ultrasound thalamotomy for essential tremor. N Engl J Med 375:730–739. https://doi.org/10.1056/NEJMoa1600159
Martínez-Fernández R, Máñez-Miró JU, Rodríguez-Rojas R et al (2020) Randomized trial of focused ultrasound subthalamotomy for parkinson’s disease. N Engl J Med 383:2501–2513. https://doi.org/10.1056/NEJMoa2016311
Song K-H, Harvey BK, Borden MA (2018) State-of-the-art of microbubble-assisted blood-brain barrier disruption. Theranostics 8:4393–4408. https://doi.org/10.7150/thno.26869
Dauba A, Delalande A, Kamimura HAS et al (2020) Recent advances on ultrasound contrast agents for blood-brain barrier opening with focused ultrasound. Pharmaceutics 12:1125. https://doi.org/10.3390/pharmaceutics12111125
Stride E, Coussios C (2019) Nucleation, mapping and control of cavitation for drug delivery. Nat Rev Phys. https://doi.org/10.1038/s42254-019-0074-y
Aryal M, Arvanitis CD, Alexander PM, McDannold N (2014) Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv Drug Deliv Rev 72:94–109. https://doi.org/10.1016/j.addr.2014.01.008
Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA (2001) Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits1. Radiology 220:640–646. https://doi.org/10.1148/radiol.2202001804
Meairs S (2015) Facilitation of drug transport across the blood-brain barrier with ultrasound and microbubbles. Pharmaceutics 7:275–293. https://doi.org/10.3390/pharmaceutics7030275
Schoen S, Kilinc MS, Lee H et al (2022) Towards controlled drug delivery in brain tumors with microbubble-enhanced focused ultrasound. Adv Drug Deliv Rev 180:114043. https://doi.org/10.1016/j.addr.2021.114043
Carpentier A, Canney M, Vignot A et al (2016) Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 8:3432. https://doi.org/10.1126/scitranslmed.aaf6086
Mainprize T, Lipsman N, Huang Y et al (2019) Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep 9:321. https://doi.org/10.1038/s41598-018-36340-0
Lipsman N, Meng Y, Bethune AJ et al (2018) Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun 9:2336. https://doi.org/10.1038/s41467-018-04529-6
Idbaih A, Canney M, Belin L et al (2019) Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin Cancer Res Clincanres. https://doi.org/10.1158/1078-0432.CCR-18-3643
Miller MW, Miller DL, Brayman AA (1996) A review of in vitro bioeffects of inertial ultrasonic cavitation from a mechanistic perspective. Ultrasound Med Biol 22:1131–1154. https://doi.org/10.1016/S0301-5629(96)00089-0
Cleve S, Inserra C, Prentice P (2019) Contrast Agent Microbubble Jetting during Initial Interaction with 200-kHz Focused Ultrasound. Ultrasound Med Biol 45:3075–3080. https://doi.org/10.1016/j.ultrasmedbio.2019.08.005
Chen H, Kreider W, Brayman AA et al (2011) Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys Rev Lett 106:034301. https://doi.org/10.1103/PhysRevLett.106.034301
Todd N, Angolano C, Ferran C et al (2020) Secondary effects on brain physiology caused by focused ultrasound-mediated disruption of the blood–brain barrier. J Controlled Release 324:450–459. https://doi.org/10.1016/j.jconrel.2020.05.040
McMahon D, O’Reilly MA, Hynynen K (2021) Therapeutic agent delivery across the blood-brain barrier using focused ultrasound. Annu Rev Biomed Eng. https://doi.org/10.1146/annurev-bioeng-062117-121238
McMahon D, Hynynen K (2017) Acute inflammatory response following increased blood-brain barrier permeability induced by focused ultrasound is dependent on microbubble dose. Theranostics 7:3989–4000. https://doi.org/10.7150/thno.21630
Kovacs ZI, Kim S, Jikaria N et al (2016) Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1614777114
Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N (2012) Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PLoS ONE 7:e45783. https://doi.org/10.1371/journal.pone.0045783
Baseri B, Choi JJ, Tung Y-S, Konofagou EE (2010) Multi-modality safety assessment of blood-brain barrier opening using focused ultrasound and definity microbubbles: a short-term study. Ultrasound Med Biol 36:1445–1459. https://doi.org/10.1016/j.ultrasmedbio.2010.06.005
McMahon D, Lassus A, Gaud E et al (2020) Microbubble formulation influences inflammatory response to focused ultrasound exposure in the brain. Sci Rep 10:21534. https://doi.org/10.1038/s41598-020-78657-9
Arvanitis CD, Vykhodtseva N, Jolesz F et al (2015) Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model. J Neurosurg 124:1450–1459. https://doi.org/10.3171/2015.4.JNS142862
McDannold N, Zhang Y, Vykhodtseva N (2016) Nonthermal ablation in the rat brain using focused ultrasound and an ultrasound contrast agent: long-term effects. J Neurosurg 125:1539–1548. https://doi.org/10.3171/2015.10.JNS151525
Peng C, Sun T, Vykhodtseva N et al (2019) Intracranial non-thermal ablation mediated by transcranial focused ultrasound and phase-shift nanoemulsions. Ultrasound Med Biol 45:2104–2117. https://doi.org/10.1016/j.ultrasmedbio.2019.04.010
Sukovich JR, Cain CA, Pandey AS et al (2018) In vivo histotripsy brain treatment. J Neurosurg. https://doi.org/10.3171/2018.4.JNS172652
Hendricks-Wenger A, Hutchison R, Vlaisavljevich E, Allen IC (2021) Immunological effects of histotripsy for cancer therapy. Front Oncol 11:681629. https://doi.org/10.3389/fonc.2021.681629
Anastasiadis P, Gandhi D, Guo Y, Ahmed A-K, Bentzen SM, Arvanitis C, Woodworth GF (2021) Localized blood–brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions–controlled focused ultrasound. Proc Natl Acad Sci 118(37):e2103280118. https://doi.org/10.1073/pnas.2103280118
Kim C, Guo Y, Leisen J et al (2021) Closed-loop trans-skull ultrasound hyperthermia leads to improved drug delivery from thermosensitive drugs and promotes changes in vascular transport dynamics in brain tumors. Theranostics 11:18
McDannold N, Vykhodtseva N, Jolesz FA, Hynynen K (2004) MRI investigation of the threshold for thermally induced blood-brain barrier disruption and brain tissue damage in the rabbit brain. Magn Reson Med 51:913–923. https://doi.org/10.1002/mrm.20060
Sabbagh A, Beccaria K, Ling X et al (2021) Opening of the blood-brain barrier using low-intensity pulsed ultrasound enhances responses to immunotherapy in preclinical glioma models. Clin Cancer Res. https://doi.org/10.1158/1078-0432.CCR-20-3760
Alkins R, Burgess A, Ganguly M et al (2013) Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res 73:1892–1899. https://doi.org/10.1158/0008-5472.CAN-12-2609
Sampson JH, Gunn MD, Fecci PE, Ashley DM (2020) Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer 20:12–25. https://doi.org/10.1038/s41568-019-0224-7
Engelhardt B, Ransohoff RM (2012) Capture, crawl, cross: the T cell code to breach the blood–brain barriers. Trends Immunol 33:579–589. https://doi.org/10.1016/j.it.2012.07.004
Schläger C, Körner H, Krueger M et al (2016) Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature 530:349–353. https://doi.org/10.1038/nature16939
Tawbi HA, Forsyth PA, Algazi A et al (2018) Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med 379:722–730. https://doi.org/10.1056/NEJMoa1805453
Long GV, Atkinson V, Lo S et al (2018) Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol 19:672–681. https://doi.org/10.1016/S1470-2045(18)30139-6
Zeng J, See AP, Phallen J et al (2013) Anti-PD-1 Blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol 86:343–349. https://doi.org/10.1016/j.ijrobp.2012.12.025
Filley AC, Henriquez M, Dey M (2017) Recurrent glioma clinical trial, CheckMate-143: the game is not over yet. Oncotarget 8:91779–91794. https://doi.org/10.18632/oncotarget.21586
Reardon DA, Brandes AA, Omuro A et al (2020) Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma. JAMA Oncol 6:1–8. https://doi.org/10.1001/jamaoncol.2020.1024
Cloughesy TF, Mochizuki AY, Orpilla JR et al (2019) Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat Med 25:477–486. https://doi.org/10.1038/s41591-018-0337-7
Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R et al (2019) Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 25:470–476. https://doi.org/10.1038/s41591-018-0339-5
O’Rourke DM, Nasrallah M, Morrissette JJ et al (2016) Pilot study of T cells redirected to EGFRvIII with a chimeric antigen receptor in patients with EGFRvIII+ glioblastoma. J Clin Oncol 34:2067–2067. https://doi.org/10.1200/JCO.2016.34.15_suppl.2067
Brown CE, Alizadeh D, Starr R et al (2016) Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 375:2561–2569. https://doi.org/10.1056/NEJMoa1610497
Ahmed N, Brawley V, Hegde M et al (2017) HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncol 3:1094–1101. https://doi.org/10.1001/jamaoncol.2017.0184
Majzner RG, Ramakrishna S, Yeom KW et al (2022) GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. https://doi.org/10.1038/s41586-022-04489-4
Arvanitis CD, Ferraro GB, Jain RK (2020) The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer 20:26–41. https://doi.org/10.1038/s41568-019-0205-x
Sarkaria JN, Hu LS, Parney IF et al (2018) Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncol 20:184–191. https://doi.org/10.1093/neuonc/nox175
Phoenix TN, Patmore DM, Boop S et al (2016) Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 29:508–522. https://doi.org/10.1016/j.ccell.2016.03.002
Vanan MI, Eisenstat DD (2015) DIPG in children – what can we learn from the past? Front Oncol. https://doi.org/10.3389/fonc.2015.00237
Lim M, Xia Y, Bettegowda C, Weller M (2018) Current state of immunotherapy for glioblastoma. Nat Rev Clin Oncol 15:422–442. https://doi.org/10.1038/s41571-018-0003-5
Rao G, Latha K, Ott M et al (2020) Anti–PD-1 Induces M1 Polarization in the glioma microenvironment and exerts therapeutic efficacy in the absence of CD8 Cytotoxic T Cells. Clin Cancer Res 26:4699–4712. https://doi.org/10.1158/1078-0432.CCR-19-4110
Gordon SR, Maute RL, Dulken BW et al (2017) PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 545:495–499. https://doi.org/10.1038/nature22396
Morad G, Helmink BA, Sharma P, Wargo JA (2021) Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. https://doi.org/10.1016/j.cell.2021.09.020
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168. https://doi.org/10.1056/NEJMra1703481
Thorsson V, Gibbs DL, Brown SD et al (2018) The immune landscape of cancer. Immunity 48:812-830.e14. https://doi.org/10.1016/j.immuni.2018.03.023
Brown CE, Aguilar B, Starr R et al (2018) Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol Ther 26:31–44. https://doi.org/10.1016/j.ymthe.2017.10.002
Srinivasan ES, Sankey EW, Grabowski MM et al (2020) The intersection between immunotherapy and laser interstitial thermal therapy: a multipronged future of neuro-oncology. Int J Hyperthermia 37:27–34. https://doi.org/10.1080/02656736.2020.1746413
Jackson CM, Kochel CM, Nirschl CJ et al (2016) Systemic tolerance mediated by melanoma brain tumors is reversible by radiotherapy and vaccination. Clin Cancer Res Off J Am Assoc Cancer Res 22:1161–1172. https://doi.org/10.1158/1078-0432.CCR-15-1516
Fukumura D, Kloepper J, Amoozgar Z et al (2018) Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 15:325–340. https://doi.org/10.1038/nrclinonc.2018.29
Quail DF, Joyce JA (2017) The Microenvironmental Landscape of Brain Tumors. Cancer Cell 31:326–341. https://doi.org/10.1016/j.ccell.2017.02.009
Stoklasek TA, Schluns KS, Lefrancois L (2006) Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol 177:6072–6080
Stephan MT, Moon JJ, Um SH et al (2010) Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med 16:1035–1041. https://doi.org/10.1038/nm.2198
Dubois S, Patel HJ, Zhang M et al (2008) Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol 180:2099–2106
Rubinstein MP, Kovar M, Purton JF et al (2006) Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc Natl Acad Sci U A 103:9166–9171. https://doi.org/10.1073/pnas.0600240103
Waldmann TA (2006) The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 6:595–601. https://doi.org/10.1038/nri1901
Kaehler KC, Piel S, Livingstone E et al (2010) Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol 37:485–498. https://doi.org/10.1053/j.seminoncol.2010.09.003
Kovacs ZI, Kim S, Jikaria N et al (2017) Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A 114:E75–E84. https://doi.org/10.1073/pnas.1614777114
Poon C, Pellow C, Hynynen K (2021) Neutrophil recruitment and leukocyte response following focused ultrasound and microbubble mediated blood-brain barrier treatments. Theranostics 11:1655–1671. https://doi.org/10.7150/thno.52710
Sinharay S, Tu T-W, Kovacs ZI et al (2019) In vivo imaging of sterile microglial activation in rat brain after disrupting the blood-brain barrier with pulsed focused ultrasound: [18F]DPA-714 PET study. J Neuroinflammation 16:155. https://doi.org/10.1186/s12974-019-1543-z
Curley CT, Stevens AD, Mathew AS et al (2020) Immunomodulation of intracranial melanoma in response to blood-tumor barrier opening with focused ultrasound. Theranostics 10:8821–8833. https://doi.org/10.7150/thno.47983
Chen P-Y, Hsieh H-Y, Huang C-Y et al (2015) Focused ultrasound-induced blood-brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med 13:93. https://doi.org/10.1186/s12967-015-0451-y
Kovacs Z, Werner B, Rassi A et al (2014) Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Controlled Release 187:74–82. https://doi.org/10.1016/j.jconrel.2014.05.033
Liu H-L, Hua M-Y, Chen P-Y et al (2010) Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 255:415–425. https://doi.org/10.1148/radiol.10090699
Liu H-L, Huang C-Y, Chen J-Y et al (2014) Pharmacodynamic and Therapeutic Investigation of Focused Ultrasound-Induced Blood-Brain Barrier Opening for Enhanced Temozolomide Delivery in Glioma Treatment. PLoS ONE. https://doi.org/10.1371/journal.pone.0114311
Sheybani ND, Breza VR, Paul S et al (2021) ImmunoPET-informed sequence for focused ultrasound-targeted mCD47 blockade controls glioma. J Controlled Release 331:19–29. https://doi.org/10.1016/j.jconrel.2021.01.023
Treat LH, McDannold N, Zhang Y et al (2012) Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med Biol 38:1716–1725. https://doi.org/10.1016/j.ultrasmedbio.2012.04.015
Wei H-J, Upadhyayula PS, Pouliopoulos AN et al (2021) Focused Ultrasound-mediated blood-brain barrier opening increases delivery and efficacy of etoposide for glioblastoma treatment. Int J Radiat Oncol 110:539–550. https://doi.org/10.1016/j.ijrobp.2020.12.019
Zhao G, Huang Q, Wang F et al (2018) Targeted shRNA-loaded liposome complex combined with focused ultrasound for blood brain barrier disruption and suppressing glioma growth. Cancer Lett 418:147–158. https://doi.org/10.1016/j.canlet.2018.01.035
Joiner JB, Pylayeva-Gupta Y (1950) Dayton PA (2020) Focused ultrasound for immunomodulation of the tumor microenvironment. J Immunol Baltim Md 205:2327–2341. https://doi.org/10.4049/jimmunol.1901430
Eranki A, Srinivasan P, Ries M et al (2020) High-intensity focused Ultrasound (HIFU) triggers immune sensitization of refractory murine neuroblastoma to checkpoint inhibitor therapy. Clin Cancer Res Off J Am Assoc Cancer Res 26:1152–1161. https://doi.org/10.1158/1078-0432.CCR-19-1604
Khokhlova V, Fowlkes J, Roberts W et al (2015) Histotripsy methods in mechanical disintegration of tissue: toward clinical applications. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group 31:145–162. https://doi.org/10.3109/02656736.2015.1007538
Hu Z, Yang XY, Liu Y et al (2007) Investigation of HIFU-induced anti-tumor immunity in a murine tumor model. J Transl Med 5:34. https://doi.org/10.1186/1479-5876-5-34
Huang X, Yuan F, Liang M et al (2012) M-HIFU inhibits tumor growth, suppresses STAT3 activity and enhances tumor specific immunity in a transplant tumor model of prostate cancer. PLoS ONE 7:e41632. https://doi.org/10.1371/journal.pone.0041632
Qu S, Worlikar T, Felsted AE et al (2020) Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy. J Immunother Cancer 8:e000200. https://doi.org/10.1136/jitc-2019-000200
Schade GR, Wang Y-N, D’Andrea S et al (2019) Boiling histotripsy ablation of renal cell carcinoma in the EKER rat promotes a systemic inflammatory response. Ultrasound Med Biol 45:137–147. https://doi.org/10.1016/j.ultrasmedbio.2018.09.006
Mathew AS, Gorick CM, Thim EA et al (2020) Transcriptomic response of brain tissue to focused ultrasound-mediated blood–brain barrier disruption depends strongly on anesthesia. Bioeng Transl Med 6:e10198. https://doi.org/10.1002/btm2.10198
Gorick CM, Mathew AS, Garrison WJ et al (2020) Sonoselective transfection of cerebral vasculature without blood–brain barrier disruption. Proc Natl Acad Sci 117:5644–5654
McMahon D, Bendayan R, Hynynen K (2017) Acute effects of focused ultrasound-induced increases in blood-brain barrier permeability on rat microvascular transcriptome. Sci Rep 7:45657. https://doi.org/10.1038/srep45657
Kovacs ZI, Tu T-W, Sundby M et al (2018) MRI and histological evaluation of pulsed focused ultrasound and microbubbles treatment effects in the brain. Theranostics 8:4837–4855. https://doi.org/10.7150/thno.24512
Rao G, Latha K, Ott M et al (2020) Anti-PD-1 induces M1 polarization in the glioma microenvironment and exerts therapeutic efficacy in the absence of CD8 cytotoxic t cells. Clin Cancer Res Off J Am Assoc Cancer Res 26:4699–4712. https://doi.org/10.1158/1078-0432.CCR-19-4110
Ilovitsh T, Feng Y, Foiret J et al (2020) Low-frequency ultrasound-mediated cytokine transfection enhances T cell recruitment at local and distant tumor sites. Proc Natl Acad Sci 117:12674–12685. https://doi.org/10.1073/pnas.1914906117
Wang J, Huang C-H, Echeagaray OH et al (2019) Microshell enhanced acoustic adjuvants for immunotherapy in glioblastoma. Adv Ther 2:1900066. https://doi.org/10.1002/adtp.201900066
Alkins R, Burgess A, Kerbel R et al (2016) Early treatment of HER2-amplified brain tumors with targeted NK-92 cells and focused ultrasound improves survival. Neuro-Oncol 18:974–981. https://doi.org/10.1093/neuonc/nov318
Kovacs ZI, Burks SR, Frank JA (2018) Focused ultrasound with microbubbles induces sterile inflammatory response proportional to the blood brain barrier opening: Attention to experimental conditions. Theranostics 8:2245–2248. https://doi.org/10.7150/thno.24181
Guo Y, Lee H, Fang Z et al (2021) Single-cell analysis reveals effective siRNA delivery in brain tumors with microbubble-enhanced ultrasound and cationic nanoparticles. Sci Adv 7:7390. https://doi.org/10.1126/sciadv.abf7390
Zhang N, Wang J, Foiret J et al (2021) Synergies between therapeutic ultrasound, gene therapy and immunotherapy in cancer treatment. Adv Drug Deliv Rev 178:113906. https://doi.org/10.1016/j.addr.2021.113906
Dewhirst MW, Lee C-T, Ashcraft KA (2016) The future of biology in driving the field of hyperthermia. Int J Hyperthermia 32:4–13. https://doi.org/10.3109/02656736.2015.1091093
Hersh DS, Kim AJ, Winkles JA et al (2016) Emerging applications of therapeutic ultrasound in neuro-oncology: moving beyond tumor ablation. Neurosurgery 79:643–654. https://doi.org/10.1227/NEU.0000000000001399
Kennedy JE (2005) High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 5:321. https://doi.org/10.1038/nrc1591
Cuenod CA, Balvay D (2013) Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging 94:1187–1204. https://doi.org/10.1016/j.diii.2013.10.010
Csoboz B, Balogh GE, Kusz E et al (2013) Membrane fluidity matters: hyperthermia from the aspects of lipids and membranes. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group 29:491–499. https://doi.org/10.3109/02656736.2013.808765
Watson KD, Lai C-Y, Qin S et al (2012) Ultrasound increases nanoparticle delivery by reducing intratumoral pressure and increasing transport in epithelial and epithelial-mesenchymal transition tumors. Cancer Res 72:1485–1493. https://doi.org/10.1158/0008-5472.CAN-11-3232
Skitzki JJ, Repasky EA (2000) Evans SS (2009) Hyperthermia as an immunotherapy strategy for cancer. Curr Opin Investig Drugs Lond Engl 10:550–558
Skandalakis GP, Rivera DR, Rizea CD et al (2020) Hyperthermia treatment advances for brain tumors. Int J Hyperthermia 37:3–19. https://doi.org/10.1080/02656736.2020.1772512
Frey B, Weiss E-M, Rubner Y et al (2012) Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 28:528–542. https://doi.org/10.3109/02656736.2012.677933
Santos MA, Wu S-K, Regenold M et al (2020) Novel fractionated ultrashort thermal exposures with MRI-guided focused ultrasound for treating tumors with thermosensitive drugs. Sci Adv 6:5684. https://doi.org/10.1126/sciadv.aba5684
Liu H-L, Hsu P-H, Lin C-Y et al (2016) Focused ultrasound enhances central nervous system delivery of bevacizumab for malignant glioma treatment. Radiology 281:99–108. https://doi.org/10.1148/radiol.2016152444
Chen P-Y, Hsieh H-Y, Huang C-Y et al (2015) Focused ultrasound-induced blood–brain barrier opening to enhance interleukin-12 delivery for brain tumor immunotherapy: a preclinical feasibility study. J Transl Med 13:93. https://doi.org/10.1186/s12967-015-0451-y
Kobus T, Zervantonakis IK, Zhang Y, McDannold NJ (2016) Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J Control Release Off J Control Release Soc 238:281–288. https://doi.org/10.1016/j.jconrel.2016.08.001
Deng J, Zhang Y, Feng J, Wu F (2010) Dendritic Cells Loaded with Ultrasound-Ablated Tumour Induce in vivo Specific Antitumour Immune Responses. Ultrasound Med Biol 36:441–448. https://doi.org/10.1016/j.ultrasmedbio.2009.12.004
Silvestrini MT, Ingham ES, Mahakian LM et al (2017) Priming is key to effective incorporation of image-guided thermal ablation into immunotherapy protocols. JCI Insight 2:e90521. https://doi.org/10.1172/jci.insight.90521
Chavez M, Silvestrini MT, Ingham ES et al (2018) Distinct immune signatures in directly treated and distant tumors result from TLR adjuvants and focal ablation. Theranostics 8:3611–3628. https://doi.org/10.7150/thno.25613
Fite BZ, Wang J, Kare AJ et al (2021) Immune modulation resulting from MR-guided high intensity focused ultrasound in a model of murine breast cancer. Sci Rep 11:927. https://doi.org/10.1038/s41598-020-80135-1
Kheirolomoom A, Silvestrini MT, Ingham ES et al (2019) Combining activatable nanodelivery with immunotherapy in a murine breast cancer model. J Control Release Off J Control Release Soc 303:42–54. https://doi.org/10.1016/j.jconrel.2019.04.008
Sheybani ND, Witter AR, Thim EA et al (2020) Combination of thermally ablative focused ultrasound with gemcitabine controls breast cancer via adaptive immunity. J Immunother Cancer 8:e001008. https://doi.org/10.1136/jitc-2020-001008
Curley CT, Sheybani ND, Bullock TN, Price RJ (2017) Focused Ultrasound immunotherapy for central nervous system pathologies: challenges and opportunities. Theranostics 7:3608–3623. https://doi.org/10.7150/thno.21225
Shin DH, Melnick KF, Tran DD, Ghiaseddin AP (2021) In situ vaccination with laser interstitial thermal therapy augments immunotherapy in malignant gliomas. J Neurooncol 151:85–92. https://doi.org/10.1007/s11060-020-03557-x
van den Bijgaart RJ, E, Eikelenboom DC, et al (2017) Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunother 66:247–258. https://doi.org/10.1007/s00262-016-1891-9
Bredlau AL, Motamarry A, Chen C et al (2018) Localized delivery of therapeutic doxorubicin dose across the canine blood–brain barrier with hyperthermia and temperature sensitive liposomes. Drug Deliv 25:973–984. https://doi.org/10.1080/10717544.2018.1461280
Salehi A, Paturu MR, Patel B et al (2020) Therapeutic enhancement of blood–brain and blood–tumor barriers permeability by laser interstitial thermal therapy. Neuro-Oncol Adv. https://doi.org/10.1093/noajnl/vdaa071
Lu P, Zhu X-Q, Xu Z-L et al (2009) Increased infiltration of activated tumor-infiltrating lymphocytes after high intensity focused ultrasound ablation of human breast cancer. Surgery 145:286–293. https://doi.org/10.1016/j.surg.2008.10.010
Xu Z-L, Zhu X-Q, Lu P et al (2009) Activation of tumor-infiltrating antigen presenting cells by high intensity focused ultrasound ablation of human breast cancer. Ultrasound Med Biol 35:50–57. https://doi.org/10.1016/j.ultrasmedbio.2008.08.005
Zhou Q, Zhu X-Q, Zhang J et al (2008) Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol 34:81–87. https://doi.org/10.1016/j.ultrasmedbio.2007.07.013
Figueroa JM, Semonche A, Magoon S et al (2020) The role of neutrophil-to-lymphocyte ratio in predicting overall survival in patients undergoing laser interstitial thermal therapy for glioblastoma. J Clin Neurosci 72:108–113. https://doi.org/10.1016/j.jocn.2019.12.057
Liu F, Hu Z, Qiu L et al (2010) Boosting high-intensity focused ultrasound-induced anti-tumor immunity using a sparse-scan strategy that can more effectively promote dendritic cell maturation. J Transl Med 8:7. https://doi.org/10.1186/1479-5876-8-7
Gamboa L, Zamat AH, Kwong GA (2020) Synthetic immunity by remote control Theranostics 10:3652–3667. https://doi.org/10.7150/thno.41305
Wu Y, Liu Y, Huang Z et al (2021) Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat Biomed Eng. https://doi.org/10.1038/s41551-021-00779-w
Meng Y, Pople CB, Budiansky D et al (2022) Current state of therapeutic focused ultrasound applications in neuro-oncology. J Neurooncol 156:49–59. https://doi.org/10.1007/s11060-021-03861-0
Schneider CS, Woodworth GF, Vujaskovic Z, Mishra MV (2020) Radiosensitization of high-grade gliomas through induced hyperthermia: Review of clinical experience and the potential role of MR-guided focused ultrasound. Radiother Oncol J Eur Soc Ther Radiol Oncol 142:43–51. https://doi.org/10.1016/j.radonc.2019.07.017
Guthkelch AN, Carter LP, Cassady JR et al (1991) Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. https://doi.org/10.1007/BF00177540
White PJ, Zhang Y-Z, Power C et al (2020) Observed effects of whole-brain radiation therapy on focused ultrasound blood-brain barrier disruption. Ultrasound Med Biol 46:1998–2006. https://doi.org/10.1016/j.ultrasmedbio.2020.04.013
Wang S, Wu C-C, Zhang H et al (2020) Focused ultrasound induced-blood–brain barrier opening in mouse brain receiving radiosurgery dose of radiation enhances local delivery of systemic therapy. Br J Radiol 93:20190214. https://doi.org/10.1259/bjr.20190214
Shi J, Fu C, Su X et al (2021) Ultrasound-stimulated microbubbles inhibit aggressive phenotypes and promotes radiosensitivity of esophageal squamous cell carcinoma. Bioengineered 12:3000–3013. https://doi.org/10.1080/21655979.2021.1931641
Acknowledgements
Costas Arvanitis research in this area is supported by the NIH (National Institutes of Health) Grant R37CA239039 (National Cancer Institute) and the Ian’s Friends Foundation. Graeme Woodworth’s research in this area is supported by NIH Grant R21NS113016 and the Focused Ultrasound Foundation
Funding
Costas Arvanitis research in this area is supported by NIH Grant R37CA239039 and the Ian’s Friends Foundation. Graeme Woodworth’s research in this area is supported by NIH Grant R21NS113016 and the Focused Ultrasound Foundation.
Author information
Authors and Affiliations
Contributions
Conceptualization: all authors; formal analysis and investigation: CK, CDA; writing—original draft preparation: CK, CDA; writing—review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, C., Lim, M., Woodworth, G.F. et al. The roles of thermal and mechanical stress in focused ultrasound-mediated immunomodulation and immunotherapy for central nervous system tumors. J Neurooncol 157, 221–236 (2022). https://doi.org/10.1007/s11060-022-03973-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-03973-1