Abstract
Objective
With an increase in the number of imaging examinations and the development of imaging technology, a small number of glioblastomas (GBMs) are identified by incidental radiological images. These incidentally discovered glioblastomas (iGBMs) are rare, and their clinical features are not well understood. Here, we investigated the clinical characteristics and outcomes of iGBM.
Methods
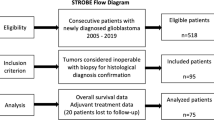
Data of newly diagnosed iGBM patients who were treated at our institution between August 2005 and October 2019 were reviewed. An iGBM was defined as a GBM without a focal sign, discovered on radiological images obtained for reasons unrelated to the tumor. Kaplan–Meier analysis was performed to calculate progression-free survival (PFS) and overall survival (OS).
Results
Of 315 patients with newly diagnosed GBM, four (1.3%) were classified as having iGBM. Health screening was the most common reason for tumor discovery (75.0%). The preoperative Karnofsky performance status score was 100 in three patients. Tumors were found on the right side in three cases. The mean volume of preoperative enhanced tumor lesion was 16.8 cm3. The median duration from confirmation of an enhanced lesion to surgery was 13.5 days. In all cases, either total (100%) or subtotal (95–99%) resections were achieved. The median PFS and OS were 10.5 and 20.0 months, respectively.
Conclusions
The iGBMs were often small and in the right non-eloquent area, and the patients had good performance status. We found that timely therapeutic intervention provided iGBM patients with favorable outcomes. This report suggests that early detection of GBM may lead to a better prognosis.


Similar content being viewed by others
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Morris Z, Whiteley WN, Longstreth WT Jr, Weber F, Lee YC, Tsushima Y, Alphs H, Ladd SC, Warlow C, Wardlaw JM, Al-Shahi Salman R (2009) Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 339:b3016. https://doi.org/10.1136/bmj.b3016
Neugut AI, Sackstein P, Hillyer GC, Jacobson JS, Bruce J, Lassman AB, Stieg PA (2019) Magnetic resonance imaging-based screening for asymptomatic brain tumors: a review. Oncologist 24:375–384. https://doi.org/10.1634/theoncologist.2018-0177
Pallud J, Fontaine D, Duffau H, Mandonnet E, Sanai N, Taillandier L, Peruzzi P, Guillevin R, Bauchet L, Bernier V, Baron MH, Guyotat J, Capelle L (2010) Natural history of incidental World Health Organization grade II gliomas. Ann Neurol 68:727–733. https://doi.org/10.1002/ana.22106
Opoku-Darko M, Lang ST, Artindale J, Cairncross JG, Sevick RJ, Kelly JJP (2018) Surgical management of incidentally discovered diffusely infiltrating low-grade glioma. J Neurosurg 129:19–26. https://doi.org/10.3171/2017.3.JNS17159
Ius T, Cesselli D, Isola M, Pauletto G, Tomasino B, D’Auria S, Bagatto D, Pegolo E, Beltrami AP, Loreto CD, Skrap M (2020) Incidental low-grade gliomas: single-institution management based on clinical, surgical, and molecular data. Neurosurgery 86:391–399. https://doi.org/10.1093/neuros/nyz114
Gogos AJ, Young JS, Pereira MP, Morshed RA, Potts MB, Hervey-Jumper SL, Berger MS (2020) Surgical management of incidentally discovered low-grade gliomas. J Neurosurg. https://doi.org/10.3171/2020.6.JNS201296
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Lamborn KR, Chang SM, Prados MD (2004) Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro Oncol 6:227–235. https://doi.org/10.1215/S1152851703000620
Weller M, Felsberg J, Hartmann C, Berger H, Steinbach JP, Schramm J, Westphal M, Schackert G, Simon M, Tonn JC, Heese O, Krex D, Nikkhah G, Pietsch T, Wiestler O, Reifenberger G, von Deimling A, Loeffler M (2009) Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 27:5743–5750. https://doi.org/10.1200/JCO.2009.23.0805
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.JNS10998
Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE (2014) Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol 32:774–782. https://doi.org/10.1200/JCO.2013.51.8886
Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, Patel AS, Rizk EB, Suki D, Sawaya R, Glantz M (2016) Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol 2:1460–1469. https://doi.org/10.1001/jamaoncol.2016.1373
Vand Rajabpour M, Yahyazadeh H, Beheshti M (2017) Prognostic factors and survival of glioblastoma multiform (GBM) in Iranian patients. Int J Cancer Manag. https://doi.org/10.5812/ijcm.6260
Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, Zhang Y, Phillips JJ, Shai A, Lafontaine M, Crane J, Chandra A, Flanigan P, Jahangiri A, Cioffi G, Ostrom Q, Anderson JE, Badve C, Barnholtz-Sloan J, Sloan AE, Erickson BJ, Decker PA, Kosel ML, LaChance D, Eckel-Passow J, Jenkins R, Villanueva-Meyer J, Rice T, Wrensch M, Wiencke JK, Oberheim Bush NA, Taylor J, Butowski N, Prados M, Clarke J, Chang S, Chang E, Aghi M, Theodosopoulos P, McDermott M, Berger MS (2020) Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol 6:495–503. https://doi.org/10.1001/jamaoncol.2019.6143
Li YM, Suki D, Hess K, Sawaya R (2016) The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg 124:977–988. https://doi.org/10.3171/2015.5.JNS142087
Arita H, Narita Y, Matsushita Y, Fukushima S, Yoshida A, Takami H, Miyakita Y, Ohno M, Shibui S, Ichimura K (2015) Development of a robust and sensitive pyrosequencing assay for the detection of IDH1/2 mutations in gliomas. Brain Tumor Pathol 32:22–30. https://doi.org/10.1007/s10014-014-0186-0
Arita H, Narita Y, Fukushima S, Tateishi K, Matsushita Y, Yoshida A, Miyakita Y, Ohno M, Collins VP, Kawahara N, Shibui S, Ichimura K (2013) Upregulating mutations in the TERT promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol 126:267–276. https://doi.org/10.1007/s00401-013-1141-6
Arita H, Yamasaki K, Matsushita Y, Nakamura T, Shimokawa A, Takami H, Tanaka S, Mukasa A, Shirahata M, Shimizu S, Suzuki K, Saito K, Kobayashi K, Higuchi F, Uzuka T, Otani R, Tamura K, Sumita K, Ohno M, Miyakita Y, Kagawa N, Hashimoto N, Hatae R, Yoshimoto K, Shinojima N, Nakamura H, Kanemura Y, Okita Y, Kinoshita M, Ishibashi K, Shofuda T, Kodama Y, Mori K, Tomogane Y, Fukai J, Fujita K, Terakawa Y, Tsuyuguchi N, Moriuchi S, Nonaka M, Suzuki H, Shibuya M, Maehara T, Saito N, Nagane M, Kawahara N, Ueki K, Yoshimine T, Miyaoka E, Nishikawa R, Komori T, Narita Y, Ichimura K (2016) A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun 4:79. https://doi.org/10.1186/s40478-016-0351-2
Ohno M, Miyakita Y, Takahashi M, Igaki H, Matsushita Y, Ichimura K, Narita Y (2019) Survival benefits of hypofractionated radiotherapy combined with temozolomide or temozolomide plus bevacizumab in elderly patients with glioblastoma aged >/= 75 years. Radiat Oncol 14:200. https://doi.org/10.1186/s13014-019-1389-7
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW (2021) The 2021 WHO Classification of tumors of the central nervous system: a summary. Neuro Oncol 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Chang SM, Parney IF, Huang W, Anderson FA, Jr., Asher AL, Bernstein M, Lillehei KO, Brem H, Berger MS, Laws ER, Glioma Outcomes Project I (2005) Patterns of care for adults with newly diagnosed malignant glioma. JAMA 293:557–564. https://doi.org/10.1001/jama.293.5.557
Morita A (2019) Value of brain dock (Brain Screening) system in Japan. World Neurosurg 127:502. https://doi.org/10.1016/j.wneu.2019.04.211
Japan C (2017) Brain tumor registry of Japan (2005–2008). Neurol Med Chir (Tokyo) 57:9–102. https://doi.org/10.2176/nmc.sup.2017-0001
Kommers I, Bouget D, Pedersen A, Eijgelaar RS, Ardon H, Barkhof F, Bello L, Berger MS, Conti Nibali M, Furtner J, Fyllingen EH, Hervey-Jumper S, Idema AJS, Kiesel B, Kloet A, Mandonnet E, Muller DMJ, Robe PA, Rossi M, Sagberg LM, Sciortino T, van den Brink WA, Wagemakers M, Widhalm G, Witte MG, Zwinderman AH, Reinertsen I, Solheim O, De Witt Hamer PC (2021) Glioblastoma surgery imaging-reporting and data system: standardized reporting of tumor volume, location, and resectability based on automated segmentations. Cancers (Basel). https://doi.org/10.3390/cancers13122854
Corballis MC (2014) Left brain, right brain: facts and fantasies. PLoS Biol 12:e1001767. https://doi.org/10.1371/journal.pbio.1001767
Awad AW, Karsy M, Sanai N, Spetzler R, Zhang Y, Xu Y, Mahan MA (2017) Impact of removed tumor volume and location on patient outcome in glioblastoma. J Neurooncol 135:161–171. https://doi.org/10.1007/s11060-017-2562-1
Simpson JR, Horton J, Scott C, Curran WJ, Rubin P, Fischbach J, Isaacson S, Rotman M, Asbell SO, Nelson JS, Weinstein AS, Nelson DF (1993) Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme Results of three consecutive radiation therapy oncology group (RTOG) clinical trials. Int J Radiation Oncol Biol Phys 26:239–244. https://doi.org/10.1016/0360-3016(93)90203-8
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15(2):1–56. https://doi.org/10.1093/neuonc/not151
Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL (2014) Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev 23:1985–1996. https://doi.org/10.1158/1055-9965.EPI-14-0275
Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, Yang R, Diplas BH, Wang Z, Greer PK, Zhu H, Wang CY, Carpenter AB, Friedman H, Friedman AH, Keir ST, He J, He Y, McLendon RE, Herndon JE 2nd, Yan H, Bigner DD (2014) Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Oncotarget 5:1515–1525. https://doi.org/10.18632/oncotarget.1765
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. https://doi.org/10.1056/NEJMoa043331
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773. https://doi.org/10.1056/NEJMoa0808710
Nonoguchi N, Ohta T, Oh JE, Kim YH, Kleihues P, Ohgaki H (2013) TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol 126:931–937. https://doi.org/10.1007/s00401-013-1163-0
McNulty SN, Schwetye KE, Ferguson C, Storer CE, Ansstas G, Kim AH, Gutmann DH, Rubin JB, Head RD, Dahiya S (2021) BRAF mutations may identify a clinically distinct subset of glioblastoma. Sci Rep 11:19999. https://doi.org/10.1038/s41598-021-99278-w
Warren PP, Lobbous M, Peeri NC, Thompson ZJ, Thompson RC, Olson JJ, LaRocca RV, Chowdhary SA, Anderson MD, Vogelbaum MA, Markert JM, Nabors LB, Egan KM (2020) Prevalence of asymptomatic glioma and implications for survival. MedRxiv. https://doi.org/10.1101/2020.04.27.20080564
Funding
None to report.
Author information
Authors and Affiliations
Contributions
Conceptualization: YN; Methodology: DK, MO, YN; Formal analysis and investigation: DK, MO, MH-K, KI; Writing—original draft preparation: DK; Writing—review and editing: MO, YN; Funding acquisition: YN; Resources: YN; Supervision: MO, YM, MT, SY, YT, MK, KI, YN.
Corresponding author
Ethics declarations
Conflict of interest
All the authors have nothing to disclose except Dr. Ichimura and Dr. Narita. Dr. Ichimura reports grants from Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., and Daiichi Sankyo Co.Ltd., outside the submitted work. Dr. Narita reports grants from Japan Agency for Medical Research and Development, Chugai Pharmaceutical co., MSD, Eisai, Toshiba, SBI pharma, Glaxo, Abbive, Ono, Stella-pharma, Ohtuka, Meiji-seika, and Daiichi-Sankyo, outside the submitted work.
Ethical approval
This study was conducted retrospectively using data obtained for clinical purposes. This study was approved by the Internal Review Board of the National Cancer Center (Approval Number: 2004–066).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11060_2021_3931_MOESM1_ESM.tiff
Supplementary file1 Figure 1 Kaplan-Meier curves for PFS (A) and OS (B) in patients with symptomatic GBM (n = 211) and iGBM (n =4). The median PFS of symptomatic GBM and iGBM were 8.0 and 10.5 months (p=0.64), and OS were 16.0 and 20.0 months (p=0.49), respectively (TIFF 2706 kb)
11060_2021_3931_MOESM2_ESM.tiff
Supplementary file2 Figure 2 Kaplan-Meier curves for PFS (A) and OS (B) in patients with symptomatic GBM with MGMT-unmethylated (n = 129) and iGBM (n = 4). The median PFS of symptomatic GBM with MGMT-unmethylated and iGBM were 7.0 and 10.5 months (p=0.55), and OS were 14.0 and 20.0 months (p=0.29), respectively (TIFF 2706 kb)
Rights and permissions
About this article
Cite this article
Kawauchi, D., Ohno, M., Honda-Kitahara, M. et al. The clinical characteristics and outcomes of incidentally discovered glioblastoma. J Neurooncol 156, 551–557 (2022). https://doi.org/10.1007/s11060-021-03931-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-021-03931-3