Abstract
Introduction
First-line bevacizumab (BEV) is now available as a treatment option for glioblastoma patients with severe clinical conditions in Japan. However, the survival benefits remain controversial. To elucidate these potential survival benefits, we retrospectively analyzed survival in glioblastoma patients receiving BEV.
Methods
We analyzed survival in 120 patients with IDH-wild type glioblastoma treated from 2002 to 2018. Overall survival (OS) was assessed in three treatment era subgroups [pre-temozolomide (TMZ), TMZ, and TMZ–BEV], and the correlations of prognostic factors with survival were evaluated.
Results
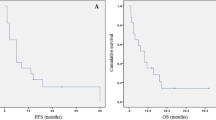
An improvement in survival was observed after BEV approval (median OS in the pre-TMZ, TMZ, and TMZ–BEV eras: 14.6, 14.9, and 22.1 months, respectively). A Cox proportional hazards model identified extent of resection and MGMT methylation status as significant prognostic factors in the TMZ era; however, these factors were not significant in the TMZ–BEV era. In subgroup analyses, patients with MGMT methylation had improved OS after TMZ introduction (pre-TMZ vs. TMZ, 18.5 vs. 28.1 months; P = 0.13), and those without MGMT methylation had significantly increased OS after BEV approval (TMZ vs. TMZ–BEV, 12.2 vs. 16.7 months; P = 0.04).
Conclusions
Our findings imply that optional first-line administration of BEV can overcome the impact of conventional risk factors and prolong survival complementary to TMZ. The patient subgroups benefitting from TMZ and BEV did not seem to overlap, and stratification based on risk factors, including MGMT methylation status, might be effective for selecting patients in whom BEV should be preferentially used as a first-line therapy.



Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Taillibert S, Kanner A et al (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318:2306–2316
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 370:709–722
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699–708
Chinot OL, Nishikawa R, Mason W et al (2016) Upfront bevacizumab may extend survival for glioblastoma patients who do not receive second-line therapy: an exploratory analysis of AVAglio. Neuro Oncol 18:1313–1318
Johnson DR, Leeper HE, Uhm JH (2013) Glioblastoma survival in the United States improved after Food and Drug Administration approval of bevacizumab: a population-based analysis. Cancer 119:3489–3495
Wachtel MS, Yang S (2014) Odds of death after glioblastoma diagnosis in the United States by chemotherapeutic era. Cancer Med 3:660–666
Zhu P, Du XL, Lu G, Zhu JJ (2017) Survival benefit of glioblastoma patients after FDA approval of temozolomide concomitant with radiation and bevacizumab: a population-based study. Oncotarget 8:44015–44031
Narita Y (2015) Bevacizumab for glioblastoma. Ther Clin Risk Manage 11:1759–1765
Yamaguchi S, Ishi Y, Motegi H et al (2018) The prognostic improvement of add-on bevacizumab for progressive disease during concomitant temozolomide and radiation therapy in the patients with glioblastoma and anaplastic astrocytoma. J Neurosurg Sci. https://doi.org/10.23736/S0390-5616.18.04463-6
Yonezawa H, Hirano H, Uchida H et al (2017) Efficacy of bevacizumab therapy for unresectable malignant glioma: a retrospective analysis. Mol Clin Oncol 6:105–110
Hata N, Yoshimoto K, Hatae R et al (2017) Add-on bevacizumab can prevent early clinical deterioration and prolong survival in newly diagnosed partially resected glioblastoma patients with a poor performance status. Onco Targets Ther 10:429–437
Hata N, Hatae R, Yoshimoto K et al (2017) Insular primary glioblastomas with IDH mutations: clinical and biological specificities. Neuropathology 37:200–206
Sturm D, Witt H, Hovestadt V et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437
Yoshimoto K, Hatae R, Sangatsuda Y et al (2017) Prevalence and clinicopathological features of H3.3 G34-mutant high-grade gliomas: a retrospective study of 411 consecutive glioma cases in a single institution. Brain Tumor Pathol 34:103–112
Watanabe T, Katayama Y, Yoshino A, Fukaya C, Yamamoto T (2005) Human interferon beta, nimustine hydrochloride, and radiation therapy in the treatment of newly diagnosed malignant astrocytomas. J Neurooncol 72:57–62
Perry JR, Laperriere N, O'Callaghan CJ et al (2017) Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med. 376:1027–1037
Arita H, Yamasaki K, Matsushita Y et al (2016) A combination of TERT promoter mutation and MGMT methylation status predicts clinically relevant subgroups of newly diagnosed glioblastomas. Acta Neuropathol Commun 4:79
Hatae R, Hata N, Suzuki SO et al (2017) A comprehensive analysis identifies BRAF hotspot mutations associated with gliomas with peculiar epithelial morphology. Neuropathology 37:191–199
Parsons DW, Jones S, Zhang X et al (2008) An integrated genomic analysis of human glioblastoma multiforme. Science 321:1807–1812
Simon M, Hosen I, Gousias K et al (2015) TERT promoter mutations: a novel independent prognostic factor in primary glioblastomas. Neuro Oncol 17:45–52
Hatae R, Hata N, Yoshimoto K et al (2016) Precise detection of IDH1/2 and BRAF hotspot mutations in clinical glioma tissues by a differential calculus analysis of high-resolution melting data. PLoS ONE 11:e0160489
Araki Y, Mizoguchi M, Yoshimoto K et al (2011) Quantitative digital assessment of MGMT immunohistochemical expression in glioblastoma tissue. Brain Tumor Pathol 28:25–31
Sandmann T, Bourgon R, Garcia J et al (2015) Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: retrospective analysis of the AVAglio trial. J Clin Oncol 33:2735–2744
Yang SB, Gao KD, Jiang T, Cheng SJ, Li WB (2017) Bevacizumab combined with chemotherapy for glioblastoma: a meta-analysis of randomized controlled trials. Oncotarget. 8:57337–57344
Acknowledgements
This work was supported by a Japanese Society for the Promotion of Science Grants-in-Aid for Scientific Research (JSPS KAKENHI) Award (Grant No. JP16K10779). We thank Ms. Aki Sako for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hata, N., Mizoguchi, M., Kuga, D. et al. First-line bevacizumab contributes to survival improvement in glioblastoma patients complementary to temozolomide. J Neurooncol 146, 451–458 (2020). https://doi.org/10.1007/s11060-019-03339-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03339-0