Abstract
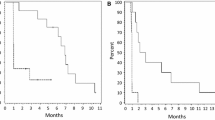
Axitinib is a small molecule tyrosine kinase inhibitor with high affinity and specificity for the family of vascular endothelial growth factor receptors. It has previously demonstrated anti-tumor activity in a small cohort of patients with recurrent glioblastoma (rGB). We conducted a non-comparative randomized phase II clinical trial investigating axitinib monotherapy versus axitinib plus lomustine (LOM) in patients with rGB. Primary endpoint was 6 month progression-free survival (6mPFS). Patients who progressed on axitinib-monotherapy were allowed to cross-over. Between August 2011 and July 2015, 79 patients were randomized and initiated axitinib monotherapy (n = 50; AXI) or axitinib plus lomustine (n = 29; AXILOM). Median age was 55y [range 18–80], 50M/28F. Baseline characteristics were well balanced between study arms. Nineteen patients in the AXI-arm crossed-over at the time of progression. Treatment was generally well tolerated. AXILOM patients were at higher risk for grade 3/4 neutropenia (0 vs. 21%) and thrombocytopenia (4 vs. 29%). Best Overall Response Rate (BORR) in the AXI-arm was 28 vs. 38% in the AXILOM-arm. 6mPFS was 26% (95% CI 14–38) versus 17% (95% CI 2–32) for patients treated in the AXI versus AXILOM-arms, respectively. Median overall survival was 29 weeks (95% CI 20–38) in the AXI-arm and 27.4 weeks (95% CI 18.4–36.5) in the AXILOM-arm. MGMT-promoter hypermethylation and steroid treatment at baseline correlated significantly with PFS and OS. We conclude from these results that axitinib improves response rate and progression-free survival in patients with rGB compared to historical controls. There is no indication that upfront combination of axitinib with LOM improves results (European Clinical Trials Database (EudraCT) Study Number: 2011-000900-16).



Similar content being viewed by others
References
De Angelis LM (2001) Brain tumors. N Engl J Med 344:114–123. doi:10.1056/NEJM200101113440207
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. doi:10.1016/S1470-2045(09)70025-7
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Lamborn KR, Yung WKA, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10:162–170. doi:10.1215/15228517-2007-062
Puputti M, Tynninen O, Sihto H et al (2006) Amplification of KIT, PDGFRA, VEGFR2, and EGFR in gliomas. Mol Cancer Res 4:927–934. doi:10.1158/1541-7786.MCR-06-0085
Reardon DA, Turner S, Peters KB et al (2011) A review of VEGF/VEGFR-targeted therapeutics for recurrent glioblastoma. J Natl Compr Canc Netw 9:414–427. doi:10.6004/jnccn.2011.0038
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740. doi:10.1200/JCO.2008.19.8721
Kreisl TN, Kim L, Moore K et al (2009) Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27:740–745. doi:10.1200/JCO.2008.16.3055
Taal W, Oosterkamp HM, Walenkamp AME et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15:943–953. doi:10.1016/S1470-2045(14)70314-6
Wick W, Brandes A, Gorlia T, Al E (2015) Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol 17 (suppl 5):v1
Escudier B, Gore M (2011) Axitinib for the management of metastatic renal cell carcinoma. Drugs R D 11:113–126. doi:10.2165/11591240-000000000-00000
Albiges L, Gizzi M, Carton E, Escudier B (2015) Axitinib in metastatic renal cell carcinoma. Expert Rev Anticancer Ther 15:499–507. doi:10.1586/14737140.2015.1033408
Lu L, Saha D, Martuza RL et al (2014) Single agent efficacy of the VEGFR kinase inhibitor axitinib in preclinical models of glioblastoma. J Neurooncol 121:91–100. doi:10.1007/s11060-014-1612-1
Kratzsch T, Gruenwald V, Vajkoczy P, Kuhn S (2013) Use of axitinib, a new-generation tyrosine kinase inhibitor, to decrease glioblastoma growth despite primary resistance to the VEGF-antibody bevacizumab. ASCO Meeting Abstracts 2013; 31 2077
Duerinck J, Du Four S, Vandervorst F et al (2016) Randomized phase II study of axitinib versus physicians best alternative choice of therapy in patients with recurrent glioblastoma. J Neurooncol 128:147–155. doi:10.1007/s11060-016-2092-2
Fleming TR (1982) One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151
Moher D, Schulz KF, Altman DG (2001) The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. The Lancet 357:1191–1194. doi:10.1016/S0140-6736(00)04337-3
Rugo HS, Herbst RS, Liu G et al (2005) Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: pharmacokinetic and clinical results. J Clin Oncol 23:5474–5483. doi:10.1200/JCO.2005.04.192
Wen PY, Macdonald DR, Reardon D a et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
National Cancer Institute (2009) Common terminology criteria for adverse events (CTCAE) v4.0
Eads CA, Danenberg KD, Kawakami K et al (2000) MethyLight: a high-throughput assay to measure DNA methylation. Nucleic Acids Res 28:E32. doi:10.1093/nar/28.8.e32
D’Haene N, Le Mercier M, De Nève N et al (2015) Clinical validation of targeted next generation sequencing for colon and lung cancers. PLoS ONE 10:e0138245. doi:10.1371/journal.pone.0138245
Le Mercier M, D’Haene N, De Nève N et al (2015) Next-generation sequencing improves the diagnosis of thyroid FNA specimens with indeterminate cytology. Histopathology 66:215–224. doi:10.1111/his.12461
Raizer JJ, Grimm S, Chamberlain MC et al (2010) A phase 2 trial of single-agent bevacizumab given in an every-3-week schedule for patients with recurrent high-grade gliomas. Cancer 116:5297–5305. doi:10.1002/cncr.25462
Duerinck J, Clement P, Bouttens F et al (2015) Patient outcome in the Belgian medical need program on bevacizumab for recurrent glioblastoma. J Neurol 262:742–751. doi:10.1007/s00415-014-7633-z
Hutterer M, Nowosielski M, Haybaeck J et al (2014) A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01–07). Neuro Oncol 16:92–102. doi:10.1093/neuonc/not161
Iwamoto FM, Lamborn KR, Robins HI et al (2010) Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro Oncol 12:855–861. doi:10.1093/neuonc/noq025
Batchelor TT, Mulholland P, Neyns B et al (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31:3212–3218. doi:10.1200/JCO.2012.47.2464
de Groot JF, Lamborn KR, Chang SM et al (2011) Phase II study of aflibercept in recurrent malignant glioma: A North American Brain Tumor Consortium Study. J Clin Oncol 29:2689–2695. doi:10.1200/JCO.2010.34.1636
Du Four S, Maenhout SK, De Pierre K et al (2015) Axitinib increases the infiltration of immune cells and reduces the suppressive capacity of monocytic MDSCs in an intracranial mouse melanoma model. Oncoimmunology 4:e998107. doi:10.1080/2162402X.2014.998107
Du Four S, Maenhout SK, Benteyn D et al (2016) Disease progression in recurrent glioblastoma patients treated with the VEGFR inhibitor axitinib is associated with increased regulatory T cell numbers and T cell exhaustion. Cancer Immunol Immunother 65:727–740. doi:10.1007/s00262-016-1836-3
Zhang X, Fang X, Gao Z et al (2014) Axitinib, a selective inhibitor of vascular endothelial growth factor receptor, exerts an anticancer effect in melanoma through promoting antitumor immunity. Anticancer Drugs 25:204–211. doi:10.1097/CAD.0000000000000033
Stehle F, Schulz K, Fahldieck C et al (2013) Reduced immunosuppressive properties of axitinib in comparison with other tyrosine kinase inhibitors. J Biol Chem 288:16334–16347. doi:10.1074/jbc.M112.437962
Bose A, Lowe DB, Rao A, Storkus WJ (2012) Combined vaccine + axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res 22:236–243. doi:10.1097/CMR.0b013e3283538293
Du Four S, Maenhout SK, Niclou SP et al (2016) Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am J Cancer Res 6:2514–2531
Larkin J, Rini BI, Nathan P et al (2016) Phase 1b dose-finding study of avelumab (anti-PD-L1)+ axitinib in treatment-naïve patients with advanced renal cell carcinoma. European Society for Medical Oncology Congress, Copenhagen, Denmark, 27 Supplement
Acknowledgements
We would like to acknowledge the patients who consented to participate in this study, their families and professional health care providers. Pfizer Belgium for the provision of axitinib study medication and a research grant. We would also like to thank the data manager Katrien Van den Bossche and Kathleen Mooren (UZ Brussel) for their help with the data collection and analysis, and Ludwig Van den Hove, PhD (Pfizer Belgium), for his support and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Duerinck, J., Du Four, S., Bouttens, F. et al. Randomized phase II trial comparing axitinib with the combination of axitinib and lomustine in patients with recurrent glioblastoma. J Neurooncol 136, 115–125 (2018). https://doi.org/10.1007/s11060-017-2629-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2629-z