Abstract
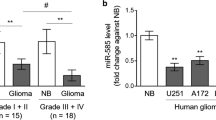
MicroRNAs are a family of small noncoding RNAs regulating gene expression by sequence-selective mRNA targeting, leading to a translational repression or mRNA degradation. The oncomiR miR-221 is highly expressed in human gliomas, as confirmed in this study in samples of low and high grade gliomas, as well in the cell lines U251, U373 and T98G. In order to alter the biological functions of miR-221, a peptide nucleic acid targeting miR-221 (R8-PNA-a221) was produced, bearing a oligoarginine peptide (R8) to facilitate uptake by glioma cells. The effects of R8-PNA-a221 were analyzed in U251, U373 and T98G glioma cells and found to strongly inhibit miR-221. In addition, the effects of R8-PNA-a221 on p27Kip1 (a target of miR-221) were analyzed in U251 and T98G cells by RT-qPCR and by Western blotting. No change of p27Kip1 mRNA content occurs in U251 cells in the presence of PNA-a221 (lacking the R8 peptide), whereas significant increase of p27Kip1 mRNA was observed with the R8-PNA-a221. These data were confirmed by Western blot assay. A clear increment of p27Kip1 protein expression in the samples treated with R8-PNA-a221 was detected. In addition, R8-PNA-a221 was found able to increase TIMP3 expression (another target of miR-221) in T98G cells. These results suggest that PNAs against oncomiRNA miR-221 might be proposed for experimental treatment of human gliomas.






Similar content being viewed by others
Abbreviations
- PNA:
-
Peptide nucleic acid
- Fl:
-
Fluorescein
- RT-qPCR:
-
Reverse transcription quantitative polymerase-chain reaction
- SDS:
-
Sodium dodecylsulphate
- SDS-PAGE:
-
SDS-polyacrylamide gel electrophoresis
- TMZ:
-
Temozolomide
- PUMA:
-
p53-upregulated modulator of apoptosis
- PTEN:
-
Phosphatase and tensin homolog
- TIMP3:
-
Metalloproteinase inhibitor 3
- ICAM-1:
-
Intercellular adhesion molecule 1
- SPR:
-
Surface plasmon resonance
- HBS:
-
Hepes buffered saline
- Ab:
-
Antibody
References
Nielsen PE, Egholm M, Berg RH, Buchardt O (1991) Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science 254:1497–1500
Nielsen PE (2001) Targeting double stranded DNA with peptide nucleic acid (PNA). Curr Med Chem 8:545–550
Borgatti M, Lampronti I, Romanelli A, Pedone C, Saviano M et al (2003) Transcription factor decoy molecules based on a peptide nucleic acid (PNA)-DNA chimera mimicking Sp1 binding sites. J Biol Chem 278:7500–7509
Gambari R (2001) Peptide-nucleic acids (PNAs): a tool for the development of gene expression modifiers. Curr Pharm Des 7:1839–1862
Gambari R (2004) Biological activity and delivery of peptide nucleic acids (PNA)-DNA chimeras for transcription factor decoy (TFD) pharmacotherapy. Curr Med Chem 11:1253–1263
Nielsen PE (2010) Peptide nucleic acids (PNA) in chemical biology and drug discovery. Chem Biodivers 7:786–804
Hatamoto M, Ohashi A, Imachi H (2010) Peptide nucleic acids (PNAs) antisense effect to bacterial growth and their application potentiality in biotechnology. Appl Microbiol Biotechnol 86:397–402
Gambari R, Borgatti M, Bezzerri V, Nicolis E, Lampronti I et al (2010) Decoy oligodeoxyribonucleotides and peptide nucleic acids-DNA chimeras targeting nuclear factor kappa-B: inhibition of IL-8 gene expression in cystic fibrosis cells infected with Pseudomonas aeruginosa. Biochem Pharmacol 80:1887–1894
Pandey VN, Upadhyay A, Chaubey B (2009) Prospects for antisense peptide nucleic acid (PNA) therapies for HIV. Expert Opin Biol Ther 9:975–989
Nielsen PE (2010) Gene targeting and expression modulation by peptide nucleic acids (PNA). Curr Pharm Des 16:3118–3123
Manicardi A, Fabbri E, Tedeschi T, Sforza S, Bianchi N et al (2012) Cellular Uptakes, biostabilities and anti-miR-210 activities of chiral Arginine-PNAs in leukaemic K562 cells. Chembiochem 13:1327–1337
Fabbri E, Manicardi A, Tedeschi T, Sforza S, Bianchi N et al (2011) Modulation of the biological activity of microRNA-210 with peptide nucleic acids (PNAs). ChemMedChem 6:2192–2202
Gambari R, Fabbri E, Borgatti M, Lampronti I, Finotti A et al (2011) Targeting microRNAs involved in human diseases: a novel approach for modification of gene expression and drug development. Biochem Pharmacol 82:1416–1429
Fabani MM, Gait MJ (2008) MiR-122 targeting with LNA/2’-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. RNA 14:336–346
Fabani MM, Abreu-Goodger C, Williams D, Lyons PA, Torres AG et al (2010) Efficient inhibition of miR-155 function in vivo by peptide nucleic acids. Nucleic Acids Res 38:4466–4475
Brown PN, Yin H (2013) PNA-based microRNA inhibitors elicit anti-inflammatory effects in microglia cells. Chem Commun (Camb) 49:4415–4417
Brognara E, Fabbri E, Aimi F, Manicardi A, Bianchi N et al (2012) Peptide nucleic acids targeting miR-221 modulate p27Kip1 expression in breast cancer MDA-MB-231 cells. Int J Oncol 41:2119–2127
He L, Hannon GJ (2010) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5:522–531
Piva R, Spandidos DA, Gambari R (2013) From microRNA functions to microRNA therapeutics: novel targets and novel drugs in breast cancer research and treatment. Int J Oncol 43:985–994
Shah MY, Calin GA (2011) MicroRNAs miR-221 and miR-222: a new level of regulation in aggressive breast cancer. Genome Med 3:56
Lambertini E, Lolli A, Vezzali F, Penolazzi L, Gambari R, Piva R (2012) Correlation between Slug transcription factor and miR-221 in MDA-MB-231 breast cancer cells. BMC Cancer 12:445
Galardi S, Mercatelli N, Giorda E, Massalini S, Frajese GV et al (2007) MiR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem 282:2316–2324
Zhang C, Zhang J, Hao J, Shi Z, Wang Y et al (2012) High level of miR-221/222 confers increased cell invasion and poor prognosis in glioma. J Transl Med 10:119
Zhang CZ, Zhang JX, Zhang AL, Shi ZD, Han L et al (2010) MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol Cancer 9:229
Lakomy R, Sana J, Hankeova S, Fadrus P, Kren L et al (2011) MiR-195, miR-196b, miR-181c, miR-21 expression levels and O-6-methylguanine-DNA methyltransferase methylation status are associated with clinical outcome in glioblastoma patients. Cancer Sci 102:2186–2190
Le Sage C, Nagel R, Egan DA, Schrier M, Mesman E (2007) Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J 26:3699–3708
Lorimer IA (2009) Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle 8:2685
Bhatia B (2010) On the move: p27Kip1 drives cell motility in glioma cells. Cell Cycle 9:1231–1240
Lu X, Zhao P, Zhang C, Fu Z, Chen Y et al (2009) Analysis of miR-221 and p27 expression in human gliomas. Mol Med Rep 2:651–656
Gillies JK, Lorimer IA (2007) Regulation of p27Kip1 by miRNA 221/222 in glioblastoma. Cell Cycle 6:2005–2009
Ueda R, Kohanbash G, Sasaki K, Fujita M, Zhu X et al (2009) Dicer-regulated microRNAs 222 and 339 promote resistance of cancer cells to cytotoxic T-lymphocytes by down-regulation of ICAM-1. Proc Natl Acad Sci USA 106:10746–10751
Zerrouqi A, Pyrzynska B, Febbraio M, Brat DJ, Van Meir EG (2012) P14ARF inhibits human glioblastoma-induced angiogenesis by upregulating the expression of TIMP3. J Clin Invest 122:1283–1295
Wang Y, Wang X, Zhang J, Sun G, Luo H et al (2012) MicroRNAs involved in the EGFR/PTEN/AKT pathway in gliomas. J Neurooncol 106:217–224
Zhang C, Kang C, You Y, Pu P, Yang W (2009) Co-suppression of miR-221/222 cluster suppresses human glioma cell growth by targeting p27Kip1 in vitro and in vivo. Int J Oncol 34:1653–1660
Schiappacassi M, Lovat F, Canzonieri V, Belletti B, Berton S et al (2008) p27Kip1 expression inhibits glioblastoma growth, invasion, and tumor-induced neoangiogenesis. Mol Cancer Ther 7:1164–1175
Tabu K, Ohnishi A, Sunden Y, Suzuki T, Tsuda M et al (2006) A novel function of OLIG2 to suppress human glial tumor cell growth via p27Kip1 transactivation. J Cell Sci 119:1433–1441
Cao X, Gu Y, Jiang L, Wang Y, Liu F et al (2013) A new approach to screening cancer stem cells from the U251 human glioma cell line based on cell growth state. Oncol Rep 29:1013–1018
Abdullah NA, Sallis B, Nuttall R, Schubert FR, Ahsan M et al (2012) Induction of apoptosis and reduction of MMP gene expression in the U373 cell line by polyphenolics in Aronia melanocarpa and by curcumin. Oncol Rep 28:1435–1442
Pen A, Durocher Y, Slinn J, Rukhlova M, Charlebois C et al (2011) Insulin-like growth factor binding protein 7 exhibits tumor suppressive and vessel stabilization properties in U87MG and T98G glioblastoma cell lines. Cancer Biol Ther 12:634–646
Abes R, Arzumanov A, Moulton H, Abes S, Ivanova G (2008) Arginine-rich cell penetrating peptides: design, structure–activity, and applications to alter pre-mRNA splicing by steric-block oligonucleotides. J Pept Sci 14:455–460
Jensen KK, Orum H, Nielsen PE, Nordén B (1997) Kinetics for hybridization of peptide nucleic acids (PNA) with DNA and RNA studied with the BIAcore technique. Biochemistry 36:5072–5077
Corradini R, Feriotto G, Sforza S, Marchelli R, Gambari R (2004) Enhanced recognition of cystic fibrosis W1282X DNA point mutation by chiral peptide nucleic acid probes by a surface plasmon resonance biosensor. J Mol Recognit 17:76–84
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Hermansen SK, Kristensen BW (2013) MicroRNA biomarkers in glioblastoma. J Neurooncol 114:13–23
Rothbard JB, Kreider E, Van Deusen CL, Wright L, Wylie BL (2002) Arginine-rich molecular transporters for drug delivery: role of backbone spacing in cellular uptake. J Med Chem 45:3612–3618
Qian X, Ren Y, Shi Z, Long L, Pu P et al (2012) Sequence-dependent synergistic inhibition of human glioma cell lines by combined temozolomide and miR-21 inhibitor gene therapy. Mol Pharm 9:2636–2645
Chen L, Zhang J, Han L, Zhang A, Zhang C et al (2012) Down regulation of miR-221/222 sensitizes glioma cells to temozolomide by regulating apoptosis independently of p53 status. Oncol Rep 27:854–860
Suzuki T, Wu D, Schlachetzki F, Li JY, Boado RJ (2004) Imaging endogenous gene expression in brain cancer in vivo with 111In-peptide nucleic acid antisense radiopharmaceuticals and brain drug-targeting technology. J Nucl Med 45:1766–1775
Boado RJ, Tsukamoto H, Pardridge WM (1998) Drug delivery of antisense molecules to the brain for treatment of Alzheimer’s disease and cerebral AIDS. J Pharm Sci 87:1308–1315
Sethi D, Chen CP, Jing RY, Thakur ML, Wickstrom E (2012) Fluorescent peptide-PNA chimeras for imaging monoamine oxidase A mRNA in neuronal cells. Bioconjug Chem 23:158–163
Acknowledgements
This work was supported by grants by AIRC (contract number IG13575) and by MIUR (PRIN09). We are grateful to Drs. Giuseppe Moretto, Alberto Beltramello, Antonio Iannucci, Giampietro Pinna, Mario Meglio, Antonio Nicolato, Bruno Bonetti, Maria Cristina Dechecchi, the Colleagues of the Dept. of Neurosciences and the Dept. of Pathology and Diagnostics of the Hospital of Verona for helpful discussions and to Dr. Amanda J. Neville for kindly revising the English text. Cinzia Cantù is a fellow of the Banco Popolare di Verona, Verona, Italy.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brognara, E., Fabbri, E., Bazzoli, E. et al. Uptake by human glioma cell lines and biological effects of a peptide-nucleic acids targeting miR-221. J Neurooncol 118, 19–28 (2014). https://doi.org/10.1007/s11060-014-1405-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1405-6