Abstract
The most frequent primary brain tumors, anaplastic astrocytomas (AA) and glioblastomas (GBM): tend to invasion of the surrounding brain. Histopathological studies found malignant cells in macroscopically unsuspicious brain parenchyma remote from the primary tumor, even affecting the contralateral hemisphere. In early stages, diffuse interneural infiltration with changes of the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) is suspected. The purpose of this study was to investigate the value of DTI as a possible instrument of depicting evidence of tumor invasion into the corpus callosum (CC). Preoperatively, 31 patients with high-grade brain tumors (8 AA and 23 GBM) were examined by MRI at 3 T, applying a high-resolution diffusion tensor imaging (DTI) sequence. ADC- and FA-values were analyzed in the tumor-associated area of the CC as identified by fiber tracking, and were compared to matched healthy controls. In (MR-)morphologically normal appearing CC the ADC values were elevated in the tumor patients (n = 22; 0.978 × 10−3 mm²/s) compared to matched controls (0.917 × 10−3 mm²/s, p < 0.05), and the corresponding relative FA was reduced (rFA: 88 %, p < 0.01). The effect was pronounced in case of affection of the CC visible on MRI (n = 9; 0.978 × 10−3 mm²/s, p < 0.05; rFA: 72 %, p < 0.01). Changes in diffusivity and anisotropy in the CC can be interpreted as an indicator of tumor spread into the contralateral hemisphere not visible on conventional MRI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) plays a central role in the staging and treatment of patients with brain tumors. While conventional MRI with T2, FLAIR, and contrast-enhanced T1-weighted sequences unravel the size, shape, and structure of lesions, the use of perfusion- and diffusion-weighted MRI adds information on the microstructural architecture and regional blood flow.
The treatment of anaplastic astrocytomas and glioblastomas first comprises a maximum surgical resection [1], which is to be followed by radiation and chemotherapy. Although affected patients often show only a small tumor burden and advanced imaging methods contribute to an adequate diagnosis and tumor localization, higher-grade astrocytomas are still considered incurable even when well accessible for surgical and radiotherapeutic treatment [2]. The palliative treatment aims at maintaining the quality of life with smallest neurological-cognitive deficits as long as possible [3]. However, in these patients the median survival time is less than 2 years, and despite of general advances in diagnosis and therapy, this poor prognosis has improved only marginally during the past 30 years [4, 5].
Histopathological studies have reported the infiltrative growth of glioblastoma multiforme (GBM) cells in apparently unaffected brain regions remote from the confirmed primary lesion [6–9] without correlating changes on computed tomographic or MR images [10, 11]. Localized proton MR spectroscopy (MRS) gives insights into tissue composition and intracellular metabolism and already provides subtle hints about remote infiltration by GBM cells [12–14]. In early tumor stages, diffuse interneural infiltration with changes of the apparent diffusion coefficient (ADC) and fractional anisotropy (FA) is suspected. Pertinent alterations were indeed observed by diffusion tensor imaging (DTI) and/or MRS of normal appearing white matter in the hemisphere contralateral to the actual glioma [12, 13].
In analogy to a previous case report [15] the purpose of this study was to investigate the value of DTI as a possible instrument of depicting evidence of tumor invasion into the corpus callosum (CC).
Materials and methods
Patients
The local ethics committee approved this work according to the declaration of Helsinki. Written informed consent was obtained from all patients and control subjects. Over a time period of 2 years pre-operative DTI was prospectively performed in all consecutive cases of suspected brain tumor before treatment. Only patients with post-operative definite histopathological confirmation of a high grade brain tumor (anaplastic astrocytoma WHO III° or glioblastoma WHO IV°) were included. Healthy age- and sex-matched volunteers without any relevant medical history served as normal controls. Data analyses were performed retrospectively.
MRI
All studies were performed on the same 3T MR system (Magnetom Trio, Siemens Medical Solutions, Erlangen/Germany). Anatomical imaging included three-dimensional (3D) T1-weighted (T1w) fast low-angle shot (FLASH) MRI (time to repetition TR 11 ms; time to echo TE 4.9 ms; flip angle 15°; isotropic image resolution 1 × 1 × 1 mm3). Diffusion tensor imaging employed a single-shot stimulated echo acquisition mode (STEAM) sequence with 24 different diffusion gradient directions (b-value 1,000 s/mm², 38 axial sections, 3 acquisitions) at 2.2 × 2.2 × 2.2 mm³ image resolution. For further details see [15].
Image analysis
The image analyses were performed retrospectively. During post-processing the diffusion-weighted images were smoothed with a 3D Gaussian filter (σ = 2.2 mm). The diffusion tensor was then calculated by a linear least-squares algorithm using an in-house software (DeffCon [16]). The tumor area was determined utilizing the clinical MR study comprising FLAIR, T2, T1 before and after gadolinium injection as well as perfusion and diffusion weighted images and manually defined on the 3D T1w datasets, and served as starting point for a fiber tracking algorithm. The fiber tracts crossing the CC were utilized to determine the region of the CC for the FA and ADC analysis. In detail, fiber assignments were estimated from the 3D FA values by a continuous axonal projection tracking algorithm (FACT [17]). This algorithm terminated the tracking of an individual fiber at FA-values below 0.15, or if the local main diffusion tensor direction deviated more than 40° in consecutive tracking steps [16]. In every subject, average FA- and ADC-values were quantified from the midsagittal plane and both adjacent slices in the affected parts of the CC, as localized by tractography.
To account for differences in absolute anisotropy in the CC sections [16] and to facilitate group comparisons irrespective of the CC section affected, the FA data were normalized by correlating to the individual FA-values of the accordant CC section of the age- and sex-matched control, referred to as relative fractional anisotropy (rFA).
Statistical analyses
Three groups were established according to the comprehensive analysis of the clinical MR study performed by two experienced neuroradiologists in consensus and, additional the medical history for group 3:
-
1. Tumor patients with obvious affection of the CC on MRI (CC-affect),
-
2. Tumor patients without obvious affection of the CC on MRI (CC-normal),
-
3. Age- and sex-matched healthy subjects in a control group (control).
The ADC- and rFA-values from the patients and subjects were analyzed by a paired student’s t test. The patients “CC-affect” and “CC-normal” were analyzed by an unpaired student’s t test.
A survival analysis was performed by applying a log-rank (Mantel-Cox) test, comparing the five patients with the highest ADC-values to the five patients with the lowest ADC-values. p-values <0.05 were regarded as significant.
Results
Demographic data
31 patients fulfilled the inclusion criteria and completed the DTI measurement: 12 females, 19 males; mean age 56.9 years, range [22–77 years]. The post-operative neuropathological diagnosis was anaplastic astrocytoma (WHO III°) in 8 cases and glioblastoma multiforme (WHO IV°) in 23 cases. DTI datasets of healthy age- and sex-matched volunteers served as control (mean age 58.7 years, range [23–81 years]).
Apparent diffusion coefficient
In the control subjects the ADC-values showed a non-significant trend to increase with age (r = 0.31, p = 0.14). Compared to the healthy controls, the ADC-values in the tumor patients rendered more inhomogeneous results, specifically if the CC was affected according to MRI (CC-affect). The patients’ ADC-values were significantly higher relative to controls (p < 0.05) (Table 1). Within the patient group, the presence of CC infiltration had no effect on the ADC-values, i.e. the average ADC-values in CC-affect or CC-normal patients were almost similar (0.97769 × 10−3 vs 0.97786 × 10−3 mm²/s; p = 0.498).
Relative fractional anisotropy
Regarding normal controls and the CC areas of patients not related to the tumor FA values significantly decreased with age (r = −0.59, p < 0.01).
There was a significant rFA reduction in the tumor corresponding region of the CC of both patient groups compared to controls (Table 2) and comparing the patients with CC affection to those with inconspicuous CC (Fig. 1).
Survival data
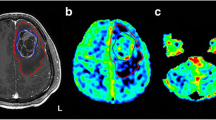
There was no significant difference between patients with CC infiltration compared to those without (median survival time: CC-affect 335.0 days vs CC-normal 358.5 days; p > 0.05). However, among the patients without CC infiltration the five patients with the lowest ADC-values tended to live longer than the five patients with the highest ADC-values (Fig. 2); median survival time: “ADC high” 157.0 days, “ADC low” 413.0 days; p < 0.05. There was no significant difference between the two groups regarding tumor size, resection or therapy.
Illustrative case
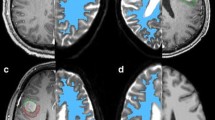
In order to illustrate possible infiltration patterns of malignant brain tumors, Fig. 3 shows pre-operative and follow-up T1-weighted MRI of a 65 year-old female with newly diagnosed GBM. At first, tumor growth was limited to the right hemisphere and fiber-tracking revealed tumor-associated nerve fibers crossing the trunk of the CC as shown below. With the tumor growth progressing despite surgery and concomitant radio-chemotherapy, post-operative follow-up examination displayed enhancement of contrast agent in those regions of the CC which showed to contain fibers connecting the primary focus with the contralateral hemisphere 10 month before.
The 3D-fiber tracts are projected on the sagittal midline 2D-image from the T1 dataset demonstrating the localization of the passage of the CC (a). The fiber tracts originating from a glioblastoma crossing the truncus of the CC—marked in solid red (b)—identify an area mainly in region 2 [16] marked in light blue (b)—Without any abnormal signal or contrast enhancement (c). The follow-up examination 12 months later reveals recurrent tumor infiltrating of the CC with contrast enhancement in the previously identified area (d)
Discussion
Only about 2 % of patients with high-grade brain tumors survive the first 5 years after diagnosis [18]. One of the main factors for the poor prognosis of glioblastomas is the invasiveness of malignant cells [19]. Histopathological studies showed glioma cells remote [6, 10] from the histologically identical main tumor bulk [9] only to differ in growth rate. These infiltrating glioma cells can hardly be imaged by standard techniques [11]. However, diffusion and metabolic abnormalities detected by DWI/DTI and proton MRS were found outside the borders of malignant brain tumors defined by conventional MRI as potential indicators of tumor infiltration or malignant transformation [12, 13, 20].
A number of effects may account for the increase of ADC-values in the section of the CC corresponding to the tumor location: An interaction of tumor cells with the extracellular matrix (ECM) is mandatory to enable cell migration. The invasiveness depends on destruction of ECM components as well as on penetration between adjacent normal brain structures [21]. Glioma cells initially infiltrate or spread micro-invasively between and around neurons and penetrate into the fiber tracts of white matter [22], causing a local displacement of the normal parenchyma without neuronal damage [23]. Then the local brain tissue is untightened and displaced under the influence of proteases [24]. Furthermore, the growth of a tumor in the CNS is associated with a disturbance of the blood–brain barrier, which leads to a vasogenic edema and an increase in parenchymal water content, which in turn increases the extracellular space and the corresponding ADC-value [25].
While disease advances, nerve cells may be destroyed and replaced by tumor cells [22]. This results in a decrease of ADC-values [26] and explains the ADC inhomogeneities among cases where MRI depicted infiltration of the CC. Regarding fractional anisotropy, a negative correlation between FA values versus cellular density and rate of tumor infiltration has been reported [27, 28], corresponding to our findings.
Abnormal FA- or ADC-values support [29] but do not prove glioma invasion since ADC cannot definitely differentiate between tumor and edema [30]. Corresponding to our data, however: tumor recurrence frequently occurs in areas of previously enhanced diffusivity, especially if fiber tracking suggests a connection to the tumor [31]. Unfortunately, only a small number of patients survived long enough to be monitored for consecutive tumor infiltration into the CC.
Limitations
Age- and sex-matched healthy individuals served as controls to minimize potential influences on DTI values [32, 33].
According to ethical considerations no specimen from normal appearing parenchyma of the corpus callosum was collected for histopathological analysis.
Conclusion
In patients with malignant gliomas brain tumors, analyzing the CC which connects both hemispheres is of major importance, since its infiltration indicates tumor spread into the contralateral hemisphere which in turn downgrades the prognosis. Our data suggest that changes in ADC and in FA in the tumor corresponding areas of the CC may be an early indicator for tumor cell infiltration in those areas. Larger clinical follow-up studies are required to confirm this postulation, because it may have a large impact on prognosis and therapy.
References
DeAngelis LM (2001) Brain tumors. N Engl J Med 344:114–123
Geer CP, Grossman SA (1997) Interstitial fluid flow along white matter tracts: a potentially important mechanism for the dissemination of primary brain tumors. J Neurooncol 32:193–201
Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY (2003) Primary brain tumours in adults. Lancet 361:323–331
Cohen BA, Knopp EA, Rusinek H, Babb JS, Zagzag D, Gonen O (2005) Assessing global invasion of newly diagnosed glial tumors with whole-brain proton MR spectroscopy. Am J Neuroradiol 26:2170–2177
Stummer W, Nestler U, Stockhammer F, Krex D, Kern BC, Mehdorn HM, Vince GH, Pichlmeier U (2010) Favorable outcome in the elderly cohort treated by concomitant temozolomide radiochemotherapy in a multicentric phase II safety study of 5-ALA. J Neurooncol 103:361–370
Matsukado Y, Maccarty CS, Kernohan JW (1961) The growth of glioblastoma multiforme (astrocytomas, grades 3 and 4) in neurosurgical practice. J Neurosurg 18:636–644
de Bouard S, Christov C, Guillamo JS, Kassar-Duchossoy L, Palfi S, Leguerinel C, Masset M, Cohen-Hagenauer O, Peschanski M, Lefrancois T (2002) Invasion of human glioma biopsy specimens in cultures of rodent brain slices: a quantitative analysis. J Neurosurg 97:169–176
Kageji T, Nagahiro S, Uyama S, Mizobuchi Y, Toi H, Nakamura M, Nakagawa Y (2004) Histopathological findings in autopsied glioblastoma patients treated by mixed neutron beam BNCT. J Neuro-Oncol 68:25–32
Silbergeld DL, Chicoine MR (1997) Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg 86:525–531
Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ (1987) Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66:865–874
Watanabe M, Tanaka R, Takeda N (1992) Magnetic-resonance-imaging and histopathology of cerebral gliomas. Neuroradiology 34:463–469
Kallenberg K, Bock HC, Helms G, Jung K, Wrede A, Buhk JH, Giese A, Frahm J, Strik H, Dechent P, Knauth M (2009) Untreated glioblastoma multiforme: increased myo-inositol and glutamine levels in the contralateral cerebral hemisphere at proton MR spectroscopy. Radiology 253:805–812
Inglese M, Brown S, Johnson G, Law M, Knopp E, Gonen O (2006) Whole-brain N-acetylaspartate spectroscopy and diffusion tensor imaging in patients with newly diagnosed gliomas: a preliminary study. AJNR Am J Neuroradiol 27:2137–2140
Busch M, Liebenrodt K, Gottfried S, Weiland E, Vollmann W, Mateiescu S, Winter S, Lange S, Sahinbas H, Baier J, van Leeuwen P, Gronemeyer D (2011) Influence of brain tumors on the MR spectra of healthy brain tissue. Magn Reson Med 65:18–27
Price SJ, Pena A, Burnet NG, Pickard JD, Gillard JH (2004) Detecting glioma invasion of the corpus callosum using diffusion tensor imaging. Br J Neurosurg 18:391–395
Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32:989–994
Mori S, Crain BJ, Chacko VP, van Zijl PC (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269
McLendon RE, Halperin EC (2003) Is the long-term survival of patients with intracranial glioblastoma multiforme overstated? Cancer 98:1745–1748
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21:1624–1636
Kimura T, Ohkubo M, Igarashi H, Kwee IL, Nakada T (2007) Increase in glutamate as a sensitive indicator of extracellular matrix integrity in peritumoral edema: a 3.0 T proton magnetic resonance spectroscopy study. J Neurosurg 106:609–613
Tysnes BB, Mahesparan R (2001) Biological mechanisms of glioma invasion and potential therapeutic targets. J Neurooncol 53:129–147
Scherer HJ (1938) Structural development in gliomas. Am J Cancer 34:333–351
Price SJ, Burnet NG, Donovan T, Green HA, Pena A, Antoun NM, Pickard JD, Carpenter TA, Gillard JH (2003) Diffusion tensor imaging of brain tumours at 3T: a potential tool for assessing white matter tract invasion? Clin Radiol 58:455–462
Demuth T, Berens ME (2004) Molecular mechanisms of glioma cell migration and invasion. J Neurooncol 70:217–228
Kono K, Inoue Y, Nakayama K, Shakudo M, Morino M, Ohata K, Wakasa K, Yamada R (2001) The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 22:1081–1088
Hartmann M, Junkers R, Herold-Mende C, Ahmadi R, Heiland S (2005) Pseudonormalization of diffusion weighted images: magnetic resonance imaging in an animal model (C6-glioma). Rofo 177:114–118
Stadlbauer A, Ganslandt O, Buslei R, Hammen T, Gruber S, Moser E, Buchfelder M, Salomonowitz E, Nimsky C (2006) Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology 240:803–810
Schluter M, Stieltjes B, Hahn HK, Rexilius J, Konrad-verse O, Peitgen HO (2005) Detection of tumour infiltration in axonal fibre bundles using diffusion tensor imaging. Int J Med Robot 1:80–86
Price SJ, Jena R, Burnet NG, Hutchinson PJ, Dean AF, Pena A, Pickard JD, Carpenter TA, Gillard JH (2006) Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol 27:1969–1974
Server A, Kulle B, Maehlen J, Josefsen R, Schellhorn T, Kumar T, Langberg CW, Nakstad PH (2009) Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema. Acta Radiol 50:682–689
Krishnan AP, Asher IM, Davis D, Okunieff P, O’Dell WG (2008) Evidence that MR diffusion tensor imaging (tractography) predicts the natural history of regional progression in patients irradiated conformally for primary brain tumors. Int J Radiat Oncol Biol Phys 71:1553–1562
Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM (2004) Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage 21:1174–1181
Hsu JL, Leemans A, Bai CH, Lee CH, Tsai YF, Chiu HC, Chen WH (2008) Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage 39:566–577
Acknowledgments
The authors (KK, JHB and PD) were supported by the Volkswagen Stiftung (Grants ZN1635 and ZN 2193).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kallenberg, K., Goldmann, T., Menke, J. et al. Glioma infiltration of the corpus callosum: early signs detected by DTI. J Neurooncol 112, 217–222 (2013). https://doi.org/10.1007/s11060-013-1049-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-013-1049-y