Abstract
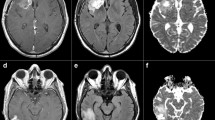
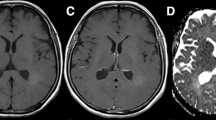
Altered diffusion in the normal appearing white matter (NAWM) of glioma patients has been explained by tumor infiltration. The goal of the present study was to test this explanation indirectly by examining whether these alterations were also present in the contralateral NAWM of non-infiltrative tumors like meningiomas; and to search for other possible reasons for this abnormality. Twenty-seven patients with histologically verified glioma (grade II–IV), 22 meningioma patients and two groups of age- and sex-matched healthy controls underwent diffusion weighted imaging (DWI) on a 3T MR. All patients were examined before treatment. Apparent diffusion coefficient (ADC) values were calculated in the entire NAWM of the hemisphere contralateral to the tumor. ADC values of the NAWM were compared between groups with Mann–Whitney U-test and multiple linear regression. The relations of ADC in NAWM to glioma grade and to tumor volume were also investigated. ADC values of the contralateral NAWM were significantly higher in both glioma and meningioma patients compared to controls (P = 0.0006 and 0.0099, respectively). ADC value was higher in the NAWM of high grade gliomas than in low grade gliomas (P = 0.0181) and in healthy control subjects (P = 0.0003). ADC did not depend on tumor volume in any of the patient groups. Elevated ADC in the NAWM of both glioma and meningioma patients might indicate that the effect of infiltrating tumor cells is not the only reason for the alteration as it has been previously suggested. Although the role of mass effect was not proved, other mechanisms might also contribute to ADC elevation.



Similar content being viewed by others
References
Puttick S, Bell C, Dowson N, Rose S, Fay M (2014) PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug Discov Today. doi:10.1016/j.drudis.2014.10.016
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, Knopp EA, Zagzag D (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Larsen VA, Simonsen HJ, Law I, Larsson HB, Hansen AE (2013) Evaluation of dynamic contrast-enhanced T1-weighted perfusion MRI in the differentiation of tumor recurrence from radiation necrosis. Neuroradiology 55:361–369. doi:10.1007/s00234-012-1127-4
Roy B, Gupta RK, Maudsley AA, Awasthi R, Sheriff S, Gu M, Husain N, Mohakud S, Behari S, Pandey CM, Rathore RK, Spielman DM, Alger JR (2013) Utility of multiparametric 3-T MRI for glioma characterization. Neuroradiology 55:603–613. doi:10.1007/s00234-013-1145-x
Cha S (2006) Update on brain tumor imaging: from anatomy to physiology. AJNR Am J Neuroradiol 27:475–487
Essig M, Anzalone N, Combs SE, Dorfler A, Lee SK, Picozzi P, Rovira A, Weller M, Law M (2012) MR imaging of neoplastic central nervous system lesions: review and recommendations for current practice. AJNR Am J Neuroradiol 33:803–817. doi:10.3174/ajnr.A2640
Essig M, Nguyen TB, Shiroishi MS, Saake M, Provenzale JM, Enterline DS, Anzalone N, Dorfler A, Rovira A, Wintermark M, Law M (2013) Perfusion MRI: the five most frequently asked clinical questions. AJR Am J Roentgenol 201:W495–W510. doi:10.2214/AJR.12.9544
Olson JJ, Nayak L, Ormond DR, Wen PY, Kalkanis SN, Ryken TC (2014) The role of targeted therapies in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol 118:557–599
Louis D, Ohgaki H, Wiestler O, Cavenee W, Burger P, Jouvet A, Scheithauer B, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Stupp R, Roila F, Group ObotEGW (2009) Malignant glioma: ESMO Clinical Recommendations for diagnosis, treatment and follow-up. Ann Oncol 20:iv126–iv128. doi:10.1093/annonc/mdp151
Ma C, Zhao G, Cruz MH, Siden A, Yakisich JS (2014) Translational gap in glioma research. Anticancer Agents Med Chem 14:1110–1120
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21:1624–1636. doi:10.1200/JCO.2003.05.063
Sahm F, Capper D, Jeibmann A, Habel A, Paulus W, Troost D, von Deimling A (2012) Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch Neurol 69:523–526. doi:10.1001/archneurol.2011.2910
Inglese M, Brown S, Johnson G, Law M, Knopp E, Gonen O (2006) Whole-brain N-acetylaspartate spectroscopy and diffusion tensor imaging in patients with newly diagnosed gliomas: a preliminary study. AJNR Am J Neuroradiol 27:2137–2140
Kallenberg K, Goldmann T, Menke J, Strik H, Bock HC, Mohr A, Buhk JH, Frahm J, Dechent P, Knauth M (2014) Abnormalities in the normal appearing white matter of the cerebral hemisphere contralateral to a malignant brain tumor detected by diffusion tensor imaging. Folia neuropathol/Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences 52:226–233
Cohen BA, Knopp EA, Rusinek H, Babb JS, Zagzag D, Gonen O (2005) Assessing global invasion of newly diagnosed glial tumors with whole-brain proton MR spectroscopy. AJNR Am J Neuroradiol 26:2170–2177
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841
Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30:1323–1341. doi:10.1016/j.mri.2012.05.001
Chan YH (2004) Biostatistics 201: linear regression analysis. Singapore Med J 45:55–61
Maudsley AA, Roy B, Gupta RK, Sheriff S, Awasthi R, Gu M, Husain N, Mohakud S, Behari S, Spielman DM (2013) Association of metabolite concentrations and water diffusivity in normal appearing brain tissue with glioma grade. J Neuroimaging. doi:10.1111/jon.12063
Nagy SA, Aradi M, Orsi G, Perlaki G, Kamson DO, Mike A, Komaromy H, Schwarcz A, Kovacs A, Janszky J, Pfund Z, Illes Z, Bogner P (2013) Bi-exponential diffusion signal decay in normal appearing white matter of multiple sclerosis. Magn Reson Imaging 31:286–295. doi:10.1016/j.mri.2012.07.007
Naganawa S, Sato K, Katagiri T, Mimura T, Ishigaki T (2003) Regional ADC values of the normal brain: differences due to age, gender, and laterality. Eur Radiol 13:6–11. doi:10.1007/s00330-002-1549-1
Orsi G, Aradi M, Nagy SA, Perlaki G, Trauninger A, Bogner P, Janszky J, Illés Z, Dóczi T, Pfund Z, Schwarcz A (2014) Differentiating white matter lesions in multiple sclerosis and migraine using monoexponential and biexponential diffusion measurements. J Magn Reson Imaging. doi:10.1002/jmri.24580
Bieza A, Krumina G (2012) Magnetic resonance study on fractional anisotropy and neuronal metabolite ratios in peritumoral area of cerebral gliomas. Medicina (Kaunas) 48:497–506
Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D (2004) Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 232:451–460. doi:10.1148/radiol.2322030959
Romano A, Fasoli F, Ferrante M, Ferrante L, Fantozzi LM, Bozzao A (2008) Fiber density index, fractional anisotropy, adc and clinical motor findings in the white matter of patients with glioblastoma. Eur Radiol 18:331–336. doi:10.1007/s00330-007-0740-9
Toh CH, Wong AM, Wei KC, Ng SH, Wong HF, Wan YL (2007) Peritumoral edema of meningiomas and metastatic brain tumors: differences in diffusion characteristics evaluated with diffusion-tensor MR imaging. Neuroradiology 49:489–494. doi:10.1007/s00234-007-0214-4
Lu S, Ahn D, Johnson G, Cha S (2003) Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumors. AJNR Am J Neuroradiol 24:937–941
Morita K, Matsuzawa H, Fujii Y, Tanaka R, Kwee IL, Nakada T (2005) Diffusion tensor analysis of peritumoral edema using lambda chart analysis indicative of the heterogeneity of the microstructure within edema. J Neurosurg 102:336–341. doi:10.3171/jns.2005.102.2.0336
Nassehi D (2013) Intracranial meningiomas, the VEGF-A pathway, and peritumoral brain oedema. Dan Med J 60:B4626
Tsai JC, Teng LJ, Chen CT, Hong TM, Goldman CK, Gillespie GY (2003) Protein kinase C mediates induced secretion of vascular endothelial growth factor by human glioma cells. Biochem Biophys Res Commun 309:952–960
Van Meir E, Sawamura Y, Diserens AC, Hamou MF, de Tribolet N (1990) Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res 50:6683–6688
Todo T, Adams EF, Rafferty B, Fahlbusch R, Dingermann T, Werner H (1994) Secretion of interleukin-6 by human meningioma cells: possible autocrine inhibitory regulation of neoplastic cell growth. J Neurosurg 81:394–401. doi:10.3171/jns.1994.81.3.0394
Black KL, Chen K, Becker DP, Merrill JE (1992) Inflammatory leukocytes associated with increased immunosuppression by glioblastoma. J Neurosurg 77:120–126. doi:10.3171/jns.1992.77.1.0120
Pauleit D, Langen KJ, Floeth F, Hautzel H, Riemenschneider MJ, Reifenberger G, Shah NJ, Muller HW (2004) Can the apparent diffusion coefficient be used as a noninvasive parameter to distinguish tumor tissue from peritumoral tissue in cerebral gliomas? J Magn Reson Imaging 20:758–764. doi:10.1002/jmri.20177
Rochfort KD, Cummins PM (2015) The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans 43:702–706. doi:10.1042/BST20140319
Acknowledgments
This study was supported by the following grants: Developing Competitiveness of Universities in the South Transdanubian Region (SROP-4.2.1.B-10/2/KONV-2010-0002 and SROP-4.2.2.A-11/1/KONV-2012-0017), “PTE ÁOK-KA-2013/12”, Hungarian Brain Research Program—“KTIA_13_NAP-A-II/9”, Hungarian Brain Research Program “B” (KTIA_NAP_13-2-2014-0019) and The Hungarian Research Found (OTKA PD103964).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement of Human Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
The present scientific contribution is dedicated to the 650th anniversary of the foundation of the University of Pécs, Hungary.
Rights and permissions
About this article
Cite this article
Horváth, A., Perlaki, G., Tóth, A. et al. Increased diffusion in the normal appearing white matter of brain tumor patients: is this just tumor infiltration?. J Neurooncol 127, 83–90 (2016). https://doi.org/10.1007/s11060-015-2011-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-2011-y