Abstract
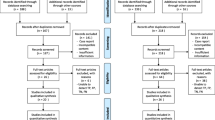
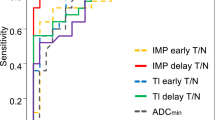
We compared pentavalent technetium-99m dimercaptosuccinic acid (Tc-99m (V) DMSA) brain single photon emission computed tomography (SPECT) and proton magnetic resonance spectroscopy (1H-MRS) for the detection of residual or recurrent gliomas after surgery and radiotherapy. A total of 24 glioma patients, previously operated upon and treated with radiotherapy, were studied. SPECT was acquired 2–3 h post-administration of 555–740 MBq of Tc-99m (V) DMSA. Lesion to normal (L/N) delayed uptake ratio was calculated as: mean counts of tumor ROI (L)/mean counts of normal mirror symmetric ROI (N). 1H-MRS was performed using a 1.5-T scanner equipped with a spectroscopy package. SPECT and 1H-MRS results were compared with pathology or follow-up neuroimaging studies. SPECT and 1H-MRS showed concordant residue or recurrence in 9/24 (37.5%) patients. Both were true negative in 6/24 (25%) patients. SPECT and 1H-MRS disagreed in 9 recurrences [7/9 (77.8%) and 2/9 (22.2%) were true positive by SPECT and 1H-MRS, respectively]. Sensitivity of SPECT and 1H-MRS in detecting recurrence was 88.8 and 61.1% with accuracies of 91.6 and 70.8%, respectively. A positive association between the delayed L/N ratio and tumor grade was found; the higher the grade, the higher is the L/N ratio (r = 0.62, P = 0.001). Tc-99m (V) DMSA brain SPECT is more accurate compared to 1H-MRS for the detection of tumor residual tissues or recurrence in glioma patients with previous radiotherapy. It allows early and non-invasive differentiation of residual tumor or recurrence from irradiation necrosis.




Similar content being viewed by others
References
Alexiou GA, Tsiouris S, Kyritsis AP, Voulgaris S, Argyropoulou MI, Fotopoulos AD (2009) Glioma recurrence versus radiation necrosis: accuracy of current imaging modalities. J Neurooncol 95:1–11
Perry A, Schmidt RE (2006) Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol 111:197–212
Hollingworth W, Medina LS, Lenkinski RE, Shibata DK, Bernal B, Zurakowski D, Comstock B, Jarvik JG (2006) A systematic literature review of magnetic resonance spectroscopy for the characterization of brain tumors. Am J Neuroradiol 27:1404–1411
Denoyer D, Perek N, Le Jeune N, Frere D, Dubois F (2004) Evidence that 99mTc-(V)-DMSA uptake is mediated by NaPi cotransporter type III in tumour cell lines. Eur J Nucl Med Mol Imaging 31:77–84
Denoyer D, Perek N, Le Jeune N, Cornillon J, Dubois F (2005) Correlation between 99mTc-(V)-DMSA uptake and constitutive level of phosphorylated focal adhesion kinase in an in vitro model of cancer cell lines. Eur J Nucl Med Mol Imaging 32:820–827
Tsiouris S, Pirmettis I, Chatzipanagiotou T, Ptohis N, Papantoniou V (2007) Pentavalent technetium-99m dimercaptosuccinic acid 99mTc-(V) DMSA brain scintitomography a plausible non-invasive depicter of glioblastoma proliferation and therapy response. J Neurooncol 85:291–295
Barai S, Bandopadhayaya G, Julka P, Malhotra A, Naik K, Haloi A, Seith A, Halanaik D (2004) Imaging of recurrent brain tumors with trivalent (99m) Tc-dimercaptosuccinic acid-initial results. Hell J Nucl Med 7:44–47
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Hirano T, Tomiyoshi K, Zhang Ying Jian, Ishida T.Inoue T, Endo K (1994) Preparation and clinical evaluation of 99mTc-DMSA for tumor scintigraphy. Eur J Nucl Med 21:82–85
Henze M, Mohammed A, Schlemmer HP, Herfarth KK, Hoffner S, Haufe S, Mier W, Eisenhut M, Debus J, Haberkorn U (2004) PET and SPECT for detection of tumor progression in irradiated low-grade astrocytoma: a receiver-operating-characteristic analysis. J Nucl Med 45:579–586
Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M (2006) Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol 2:494–503
Central Brain Tumor Registry of the United States. http://www.CBTRUS.org
Burton EC, Prados MD (2000) Malignant gliomas. Curr Treat Options Oncol 1:459–468
Byrne TN (1994) Imaging of gliomas. Semin Oncol 21:162–171
Chen W (2007) Clinical applications of PET in brain tumors. J Nucl Med 48:1468–1481
Benard F, Romsa J, Hustinx R (2002) Imaging gliomas with positron emission tomography and single-photon emission computed tomography. Semin Nucl Med 33:148–162
Soler C, Beauchesne P, Maatougui K, Schmitt T, Barral FG, Michel D, Dubois F, Brunon J (1998) Technetium-99m sestamibi brain single-photon emission tomography for detection of recurrent gliomas after radiation therapy. Eur J Nucl Med 25:1649–1657
Hirano T, Otake H, Kazama K, Wakabayashi K, Zama A, Shibasaki T, Tamura M, Endo K (1997) Technetium-99m (V)-DMSA and thallium-201 in brain tumor imaging: correlation with histology and malignant grade. J Nucl Med 38:1741–1749
Goldman S (1996) Regional glucose metabolism and histopathology of gliomas: a study based on PET-guided stereotactic biopsy. Cancer 78:1098–1106
Yoshii Y, Satou M, Yamamoto T, Yamada Y, Hyodo A, Nose T, Ishikawa H, Hatakeyama R (1993) The role of thallium-201 single photon emission tomography in the investigation and characterisation of brain tumours in man and their response to treatment. Eur J Nucl Med 20:39–45
Macapinlac H, Scott A, Caluser C et al (1992) Comparison of T1–201 and Tc-99m–2-methoxy isobutyl isonitrile (MIBI) with MRI in the evaluation of recurrent brain tumors. J Nucl Med 33:867
Vos MJ, Tony BN, Hoekstra OS, Postma TJ, Heimans JJ, Hooft L (2007) Systematic review of the diagnostic accuracy of 201 Tl single photon emission computed tomography in the detection of recurrent glioma. Nucl Med Commun 28:431–439
Kosuda S, Fujii H, Aoki S, Suzuki K, Tanaka Y, Nakamura O, Shidara N (1993) Reassessment of quantitative thallium-201 brain SPECT for miscellaneous brain tumors. Ann Nucl Med 7:257–263
Sasaki M, Ichiya Y, Kuwabara Y, Yoshida T, Inoue T, Morioka T, Hisada K, Fukui M, Masuda K (1996) Hyperperfusion and hypermetabolism in brain radiation necrosis with epileptic activity. J Nucl Med 37:1174–1176
Yoshii Y, Moritake T, Suzuki K, Fujita K, Nose T, Satou M (1996) Cerebral radiation necrosis with accumulation of thallium 201 on single-photon emission CT. ANJR Am J Neuroradiol 17:1773–1776
Le Jeune FP, Dubois F, Blond S, Steinling M (2006) Sestamibi technetium-99m brain single-photon emission computed tomography to identify recurrent glioma in adults: 201 studies. J Neurooncol 77:177–183
Borodin OYU, Velichko OB, Garganeev AB et al (2000) Comparison of 99mTc-MIBI SPECT and GD-enhanced MRI in detection of recurrent tumor in malignant. Eur J Nucl Med 27:154
Palumbo B, Lupattelli M, Pelliccioli GP, Chiarini P, Moschini TO, Palumbo I, Siepi D, Buoncristiani P, Nardi M, Giovenali P, Palumbo R (2006) Association of 99mTc-MIBI brain SPECT and proton magnetic resonance spectroscopy (1H-MRS) to assess glioma recurrence after radiotherapy. Q J Nucl Med Mol Imaging 50:88–93
Feun LG, Savaraj N, Landy HJ (1994) Drug resistance in brain tumors. J Neurooncol 20:165–176
Lehnert M (1994) Multidrug resistance in human cancer. J Neurooncol 22:239–243
Andrews DW, Das R, Kim S, Zhang J, Curtis M (1994) Technetium-MIBI as a glioma imaging agent for the assessment of multi-drug resistance. Neurosurgery 40:1323–1332
Ballinger JR, Sheldon KM, Boxen I, Erlichman C, Ling V (1995) Differences between accumulation of 99mTc-MIBI and 201Tl-thallous chloride in tumour cells: role of P-glycoprotein. Q J Nucl Med 39:122–128
Piwnica-Worms D, Chiu ML, Budding M, Kronauge JF, Kramer RA, Croop JM (1993) Functional imaging of multidrug-resistant P-glycoprotein with an organotechnetium complex. Cancer Res 53:977–984
Shibata Y, Matsumura A, Nose T (2002) Effect of expression of P-glycoprotein on technetium-99m methoxyisobutylisonitrile single photon emission computed tomography of brain tumors. Neurol Med Chir (Tokyo) 42:325–330
Hirano T, Otake H, Shibasaki T, Tamura M, Endo K (1997) Differentiating histologic malignancy of primary brain tumors: pentavalent technetium-99m-DMSA. J Nucl Med 38:20–26
Samnick S, Bader JB, Hellwig D, Moringlane JR, Alexander C, Romeike BF, Feiden W, Kirsch CM (2002) Clinical value of iodine-123-alpha-methyl-l-tyrosine single-photon emission tomography in the differential diagnosis of recurrent brain tumor in patients pretreated for glioma at follow-up. J Clin Oncol 20:396–404
Kuwert T, Woesler B, Morgenroth C, Lerch H, Schäfers M, Palkovic S, Matheja P, Brandau W, Wassmann H, Schober O (1998) Diagnosis of recurrent glioma with SPECT and iodine-123-alpha-methyl tyrosine. J Nucl Med 39:23–27
Kim EE, Chung SK, Haynie TP, Kim CG, Cho BJ, Podoloff DA, Tilbury RS, Yang DJ, Yung WK, Moser RP Jr, Ajani JA (1992) Differentiation of residual or recurrent tumors from post-treatment changes in F-18 FDG PET. Radiographics 12:269–279
Valk PE, Budinger TF, Levin VA, Silver P, Gutin PH, Doyle WK (1988) PET of malignant cerebral tumors after interstitial brachytherapy. demonstration of metabolic activity and correlation with clinical outcome. J Neurosurg 69:830–838
Glantz MJ, Hoffman JM, Coleman RE, Friedman AH, Hanson MW, Burger PC, Herndon JE 2nd, Meisler WJ, Schold SC Jr (1991) Identification of early recurrence of primary central nervous system tumors by [18F]fluorodeoxyglucose positron emission tomography. Ann Neurol 29:347–355
Ogawa T, Kanno I, Shishido F, Inugami A, Higano S, Fujita H, Murakami M, Uemura K, Yasui N, Mineura K et al (1991) Clinical value of PET with [18F]fluorodeoxyglucose and L-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. Acta Radiol 32:197–202
Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP (1998) Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? Am J Neuroradiol 19:407–413
Olivero WC, Dulebohn SC, Lister JR (1995) The use of PET in evaluating patients with primary brain tumors: is it useful? J Neurol Neurosurg Psychiatry 58:250–252
Mochizuki T, Tsukamoto E, Kuge Y, Kanegae K, Zhao S, Hikosaka K, Hosokawa M, Kohanawa M, Tamaki N (2001) FDG uptake and glucose transporter subtype expressions in experimental tumor and inflammation models. J Nucl Med 42:1551–1555
Carvalho PA, Schwartz RB, Alexander E 3rd, Garada BM, Zimmerman RE, Loeffler JS, Holman BL (1992) Detection of recurrent gliomas with quantitative thallium-201/technetium-99m HMPAO single-photon emission computerized tomography. J Neurosurg 77:565–570
Byrne TN (1994) Imaging of gliomas. Semin Oncol 21:162–171
Leeds NE, Jackson EF (1994) Current imaging techniques for the evaluation of brain neoplasms. Curr Opin Oncol 6:254–261
Kallén K, Burtscher IM, Holtås S, Ryding E, Rosén I (2000) 201Thallium SPECT and 1H-MRS compared with MRI in chemotherapy monitoring of high-grade malignant astrocytomas. J Neurooncol 46:173–185
Young RJ, Ghesani MV, Kagetsu NJ, Derogatis AJ (2005) Lesion size determines accuracy of thallium-201 brain single-photon emission tomography in differentiating between intracranial malignancy and infection in AIDS patients. Am J Neuroradiol 26:1973–1979
Rock JP, Scarpace L, Hearshen D, Gutierrez J, Fisher JL, Rosenblum M, Mikkelsen T (2004) Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery 54:1111–1117; discussion 1117–9
Chong VF, Rumpel H, Aw YS, Ho GL, Fan YF, Chua EJ (1999) Temporal lobe necrosis following radiation therapy for nasopharyngeal carcinoma: 1H MR spectroscopic findings. Int J Radiat Oncol Biol Phys 45:699–705
Schlemmer HP, Bachert P, Henze M, Buslei R, Herfarth KK, Debus J, van Kaick G (2002) Differentiation of radiation necrosis from tumor progression using proton magnetic resonance spectroscopy. Neuroradiology 44:216–222
Schlemmer HP, Bachert P, Herfarth K, Zuna I, Debus J, van Kaick G (2001) Proton MR spectroscopic evaluation of suspicious brain lesions after stereotactic radiotherapy. Am J Neuroradiol 22:1316–1324
Chong VF, Rumpel H, Fan YF, Mukherji SK (2001) Temporal lobe changes following radiation therapy: imaging and proton MR spectroscopic findings. Eur Radiol 11:317–324
Ando K, Ishikura R, Nagami Y, Morikawa T, Takada Y, Ikeda J, Nakao N, Matsumoto T, Arita N (2004) Usefulness of Cho/Cr ratio in proton MR spectroscopy for differentiating residual/recurrent glioma from non-neoplastic lesions. Nippon Igaku Hoshasen Gakkai Zasshi 64:121–126
Plotkin M, Eisenacher J, Bruhn H, Wurm R, Michel R, Stockhammer F, Feussner A, Dudeck O, Wust P, Felix R, Amthauer H (2004) 123I-IMT SPECT and 1H MR-spectroscopy at 3.0 T in the differential diagnosis of recurrent or residual gliomas: a comparative study. J Neurooncol 70:49–58
Lichy MP, Henze M, Plathow C, Bachert P, Kauczor HU, Schlemmer HP (2004) Metabolic imaging to follow stereotactic radiation of gliomas–the role of 1H MR spectroscopy in comparison to FDG-PET and IMT-SPECT. Rofo 176:1114–1121
Tzika AA, Zarifi MK, Goumnerova L, Astrakas LG, Zurakowski D, Young-Poussaint T, Anthony DC, Scott RM, Black PM (2002) Neuroimaging in pediatric brain tumors: Gd-DTPA-enhanced, hemodynamic, and diffusion MR imaging compared with MR spectroscopic imaging. Am J Neuroradiol 23:322–333
Wald LL, Nelson SJ, Day MR, Noworolski SE, Henry RG, Huhn SL, Chang S, Prados MD, Sneed PK, Larson DA, Wara WM, McDermott M, Dillon WP, Gutin PH, Vigneron DB (1997) Serial proton magnetic resonance spectroscopy imaging of glioblastoma multiforme after brachytherapy. J Neurosurg 87:525–534
McKnight TR, von dem Bussche MH, Vigneron DB, Lu Y, Berger MS, McDermott MW, Dillon WP, Graves EE, Pirzkall A, Nelson SJ (2002) Histopathological validation of a three-dimensional magnetic resonance spectroscopy index as a predictor of tumor presence. J Neurosurg 97:794–802
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amin, A., Moustafa, H., Ahmed, E. et al. Glioma residual or recurrence versus radiation necrosis: accuracy of pentavalent technetium-99m-dimercaptosuccinic acid [Tc-99m (V) DMSA] brain SPECT compared to proton magnetic resonance spectroscopy (1H-MRS): initial results. J Neurooncol 106, 579–587 (2012). https://doi.org/10.1007/s11060-011-0694-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0694-2