Abstract
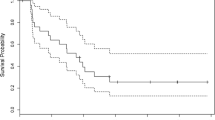
Protracted low dose temozolomide (75 mg/m2/day on days 1–21 of 28 days) offers potential advantages over standard temozolomide schedules (200 mg/m2/day on days 1–5 of 28 days) including greater cumulative drug exposure and depletion of O6-alkylguanine DNA alkyltransferase levels, theoretically overcoming intrinsic chemoresistance. We retrospectively review our experience in 25 patients with pathologically proven low grade gliomas (LGG) treated with protracted low dose temozolomide to primarily quantify its toxicity and secondarily to assess efficacy. None had previously received radiation. Tumor response was graded based on changes in tumor size, steroid requirements, and clinical exam. About 243 cycles of protracted low dose temozolomide were administered. Three patients (12%) were changed to standard temozolomide dosing due to side effects, including intractable nausea (n = 2) and multiple cytopenias (n = 1). The most frequent toxicities were fatigue (76%), lymphopenia (72% [48% high grade]), constipation (56%), and nausea (52%). High grade toxicities (other than lymphopenia) included secondary malignancy, pruritis, hyponatremia, neutropenia, leukopenia, and cognitive decline (n = 1 for each). Tumor response rate was 52% and and disease control rate was 84%. Six month PFS was 92% and 12 month PFS was 72%. Response rates and PFS were independent of pathological subtype, deletion status, and indication for chemotherapy. Protracted low dose temozolomide has a distinct spectrum of toxicities compared to standard dosing but is well tolerated in most patients and may provide improved response rates compared to standard dosing. The results of larger randomized trials are needed to assess its potential advantages over other management schemes.
Similar content being viewed by others
References
Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W, Glantz M, Greenberg H, Selker RG, Vick NA, Rampling R, Friedman H, Phillips P, Bruner J, Yue N, Osoba D, Zaknoen S, Levin VA (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593
Yung WK, Prados MD, Yaya-Tur R, Rosenfeld SS, Brada M, Friedman HS, Albright R, Olson J, Chang SM, O’Neill AM, Friedman AH, Bruner J, Yue N, Dugan M, Zaknoen S, Levin VA (1999) Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol 17:2762–2771
Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R, Stupp R, Yue N, Bruner J, Dugan M, Rao S, Zaknoen S (2001) Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol 12:259–266
van den Bent MJ, Taphoorn MJ, Brandes AA, Menten J, Stupp R, Frenay M, Chinot O, Kros JM, van der Rijt CC, Vecht Ch J, Allgeier A, Gorlia T (2003) Phase II study of first-line chemotherapy with temozolomide in recurrent oligodendroglial tumors: the European Organization for Research and Treatment of Cancer Brain Tumor Group Study 26971. J Clin Oncol 21:2525–2528
Brada M, Viviers L, Abson C, Hines F, Britton J, Ashley S, Sardell S, Traish D, Gonsalves A, Wilkins P, Westbury C (2003) Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol 14:1715–1721
Byrne TN (2004) Response of low-grade oligodendroglial tumors to temozolomide. J Neurooncol 70:279–280
Hoang-Xuan K, Capelle L, Kujas M, Taillibert S, Duffau H, Lejeune J, Polivka M, Criniere E, Marie Y, Mokhtari K, Carpentier AF, Laigle F, Simon JM, Cornu P, Broet P, Sanson M, Delattre JY (2004) Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol 22:3133–3138
Levin N, Lavon I, Zelikovitsh B, Fuchs D, Bokstein F, Fellig Y, Siegal T (2006) Progressive low-grade oligodendrogliomas: response to temozolomide and correlation between genetic profile and O6-methylguanine DNA methyltransferase protein expression. Cancer 106:1759–1765
Pace A, Vidiri A, Galie E, Carosi M, Telera S, Cianciulli AM, Canalini P, Giannarelli D, Jandolo B, Carapella CM (2003) Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol 14:1722–1726
Quinn JA, Reardon DA, Friedman AH, Rich JN, Sampson JH, Provenzale JM, McLendon RE, Gururangan S, Bigner DD, Herndon JE 2nd, Avgeropoulos N, Finlay J, Tourt-Uhlig S, Affronti ML, Evans B, Stafford-Fox V, Zaknoen S, Friedman HS (2003) Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol 21:646–651
Newlands ES, Blackledge GR, Slack JA, Rustin GJ, Smith DB, Stuart NS, Quarterman CP, Hoffman R, Stevens MF, Brampton MH et al. (1992) Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer 65:287–291
Stevens MF, Hickman JA, Langdon SP, Chubb D, Vickers L, Stone R, Baig G, Goddard C, Gibson NW, Slack JA et al. (1987) Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res 47:5846–5852
Tolcher AW, Gerson SL, Denis L, Geyer C, Hammond LA, Patnaik A, Goetz AD, Schwartz G, Edwards T, Reyderman L, Statkevich P, Cutler DL, Rowinsky EK (2003) Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer 88:1004–1011
Spiro TP, Liu L, Majka S, Haaga J, Willson JK, Gerson SL (2001) Temozolomide: the effect of once- and twice-a-day dosing on tumor tissue levels of the DNA repair protein O(6)-alkylguanine-DNA-alkyltransferase. Clin Cancer Res 7:2309–2317
Khan RB, Raizer JJ, Malkin MG, Bazylewicz KA, Abrey LE (2002) A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro-oncol 4:39–43
Brock CS, Newlands ES, Wedge SR, Bower M, Evans H, Colquhoun I, Roddie M, Glaser M, Brampton MH, Rustin GJ (1998) Phase I trial of temozolomide using an extended continuous oral schedule. Cancer Res 58:4363–4367
Pegg AE (1990) Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res 50:6119–6129
Gerson SL (2002) Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol 20:2388–2399
Silber JR, Blank A, Bobola MS, Ghatan S, Kolstoe DD, Berger MS (1999) O6-methylguanine-DNA methyltransferase-deficient phenotype in human gliomas: frequency and time to tumor progression after alkylating agent-based chemotherapy. Clin Cancer Res 5:807–814
Hermisson M, Klumpp A, Wick W, Wischhusen J, Nagel G, Roos W, Kaina B, Weller M (2006) O6-methylguanine DNA methyltransferase and p53 status predict temozolomide sensitivity in human malignant glioma cells. J Neurochem 96:766–776
Baer JC, Freeman AA, Newlands ES, Watson AJ, Rafferty JA, Margison GP (1993) Depletion of O6-alkylguanine-DNA alkyltransferase correlates with potentiation of temozolomide and CCNU toxicity in human tumour cells. Br J Cancer 67:1299–1302
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L, Bromberg JE, Hau P, Mirimanoff RO, Cairncross JG, Janzer RC, Stupp R (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
van den Bent MJ, Stupp R, Brandes AA, Lacombe D (2004) Current and future trials of the EORTC brain tumor group. Onkologie 27:246–250
Bex A, Kerst J, Mallo H, Van Tinteren H, Horenblas S, De Gast GC (2005) Extended continuous oral temozolomide in patients with progressive metastatic renal cell carcinoma not responding to interferon alpha 2b. J Chemother 17:674–678
Tosoni A, Cavallo G, Ermani M, Scopece L, Franceschi E, Ghimenton C, Gardiman M, Pasetto L, Blatt V, Brandes AA (2006) Is protracted low-dose temozolomide feasible in glioma patients? Neurology 66:427–429
Garcia del Muro X, Lopez-Pousa A, Martin J, Buesa JM, Martinez-Trufero J, Casado A, Poveda A, Cruz J, Bover I, Maurel J (2005) A phase II trial of temozolomide as a 6-week, continuous, oral schedule in patients with advanced soft tissue sarcoma: a study by the Spanish Group for Research on Sarcomas. Cancer 104:1706–1712
Macdonald DR, Cascino TL, Schold SC Jr., Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Bobola MS, Silber JR, Ellenbogen RG, Geyer JR, Blank A, Goff RD (2005) O6-methylguanine-DNA methyltransferase, O6-benzylguanine, and resistance to clinical alkylators in pediatric primary brain tumor cell lines. Clin Cancer Res 11:2747–2755
Quinn JA, Desjardins A, Weingart J, Brem H, Dolan ME, Delaney SM, Vredenburgh J, Rich J, Friedman AH, Reardon DA, Sampson JH, Pegg AE, Moschel RC, Birch R, McLendon RE, Provenzale JM, Gururangan S, Dancey JE, Maxwell J, Tourt-Uhlig S, Herndon JE 2nd, Bigner DD, Friedman HS (2005) Phase I trial of temozolomide plus O6-benzylguanine for patients with recurrent or progressive malignant glioma. J Clin Oncol 23:7178–7187
Middleton MR, Lee SM, Arance A, Wood M, Thatcher N, Margison GP (2000) O6-methylguanine formation, repair protein depletion and clinical outcome with a 4 hr schedule of temozolomide in the treatment of advanced melanoma: results of a phase II study. Int J Cancer 88:469–473
Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA, Chapman PB (2004) Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol 22:610–616
Villano JL, Collins CA, Manasanch EE, Ramaprasad C, van Besien K (2006) Aplastic anaemia in patient with glioblastoma multiforme treated with temozolomide. Lancet Oncol 7:436–438
Karim AB, Afra D, Cornu P, Bleehan N, Schraub S, De Witte O, Darcel F, Stenning S, Pierart M, Van Glabbeke M (2002) Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Int J Radiat Oncol Biol Phys 52:316–324
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pouratian, N., Gasco, J., Sherman, J.H. et al. Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. J Neurooncol 82, 281–288 (2007). https://doi.org/10.1007/s11060-006-9280-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-006-9280-4